Figure 4.
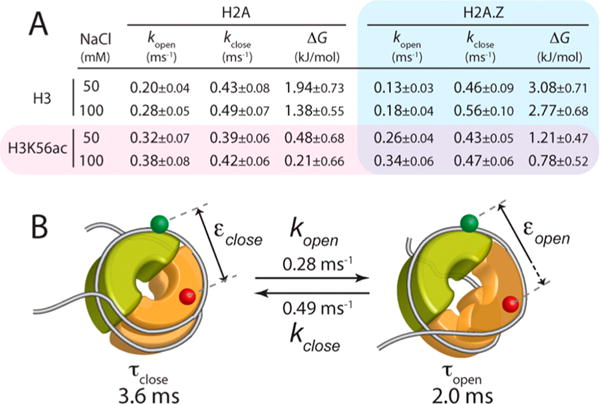
Hybrid single-molecule approach combining MLE and FCS that reveals the kinetics of the histone core dynamics at the dimer–tetramer interface. (A) Opening (kopen) and closing (kclose) rate constants, and the free energy change (ΔG) of the opening motion calculated from the rate constants for the nucleosomes made with the wild-type histone core (H2A and H3), the H3K56-acetylated histone core (H3K56ac and H3), the H2A.Z-substituted histone core (H2A.Z and H3), and the H3K56-acetylated and H2A.Z-substituted histone core (H2A.Z and H3K56ac) under two salt conditions (50 and 100 mM NaCl). (B) Quantitative summary of the histone core dynamics at the dimer–tetramer interface at 100 mM NaCl. See the legend of panels A and B of Figure 1 for the descriptions of the nucleosome structure.
