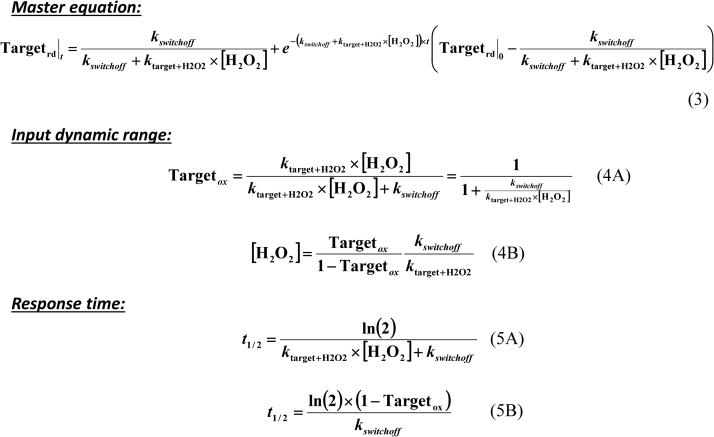
Fig. 5.
Governing equations of H2O2 signaling. The master equation (Eq. 3) includes the dependence on the sustained H2O2 signaling concentration [H2O2] attained in the vicinity of the redox switch during the signaling process, as well as the rate constants for oxidation (ktarget+H2O2) and reduction (kswitchoff) of the redox switch, and the fraction of the redox switch in the reduced form at time 0 (). The key features of H2O2 signaling are described by two sets of simpler equations deduced from the master Eq. 3 [8], [23]. Eq. 4A is deduced by letting t tend to infinite and represents the steady-state fraction of the redox switch in the oxidized form (Targetox), which is a measure of the amount of information transmitted from H2O2 to the redox target. Calculation of the H2O2 signaling concentration causing a certain steady-state value of target oxidation is done with Eq. 4B, which results from an arrangement of Eq. 4A. The second set of equations (Eqs. 5A and 5B) calculates the response time of the redox switch to H2O2, giving the time (t1/2) needed for oxidizing half of the target present initially, i.e., indicating whether transmission of information proceeds rapid enough. Eq. 5A is deduced by replacing by and t by t1/2 in Eq. 3 and calculates t1/2 as a function of H2O2 concentration. Eq. 5B is deduced by replacing Eq. 4B in Eq. 5A and calculates t1/2 as a function of the steady-state fraction of the redox switch in the oxidized form and on the value of kswitchoff.