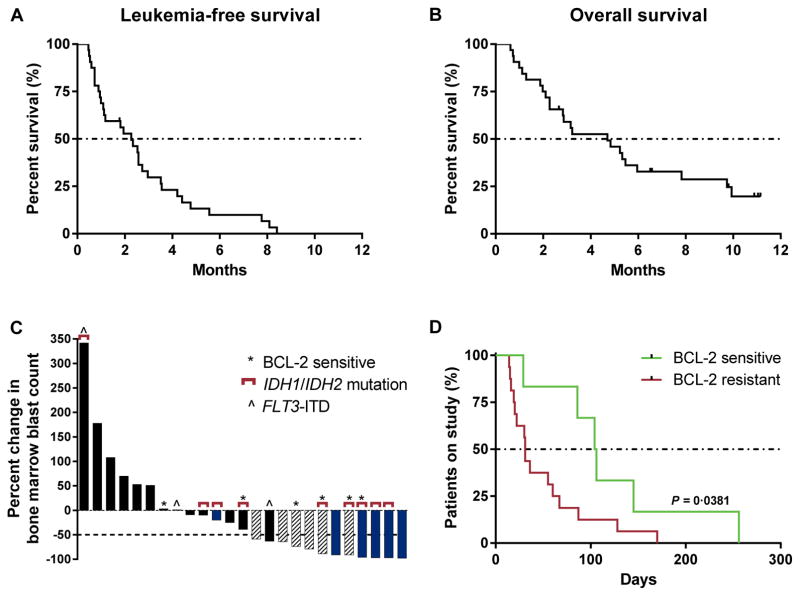
Figure 1.
Biologic activity of venetoclax. A and B, the median leukemia-free survival was 2.3 months (range 1.0–2.7) and the median overall survival was 4.7 months (range 2.3–6.0), in relapsed/refractory AML patients or those unfit for intensive chemotherapy treated with venetoclax monotherapy. C, 25 (78%) patients had bone marrow blast counts evaluable at the first assessment (week 4). The best percent change in bone marrow counts at the first assessment (week 4) is shown. Six patients came off study before the first assessment and 1 patient had unevaluable bone marrow (aplasia). Patients who achieved an objective response by the IWG criteria (complete response/complete response with incomplete blood count recovery) are indicated with blue, and patients who had anti-leukemic activity that did not meet IWG criteria are indicated with hashed bars. Patients with a BCL-2 family protein sensitive index at screening and/or IDH1/2 mutations are annotated with asterisks and brackets, respectively. D, 6 patients with a BCL-2 family sensitive index (green) achieved longer durations on venetoclax therapy than the 16 patients with a BCL-2 family resistant index (red) (P = 0.0381 by Wilcoxon signed rank test). The median number of days on venetoclax was 96 days for patients with a BCL-2 sensitive index vs 31 days for patients with a BCL-2 resistant index.