Abstract
Axonal degeneration is a pivotal feature of many neurodegenerative conditions and substantially accounts for neurological morbidity. A widely used experimental model to study the mechanisms of axonal degeneration is Wallerian degeneration (WD), which occurs after acute axonal injury. In the peripheral nervous system (PNS), WD is characterized by swift dismantling and clearance of injured axons with their myelin sheaths. This is a prerequisite for successful axonal regeneration. In the central nervous system (CNS), WD is much slower, which significantly contributes to failed axonal regeneration. Although it is well-documented that Schwann cells (SCs) have a critical role in the regenerative potential of the PNS, to date we have only scarce knowledge as to how SCs ‘sense’ axonal injury and immediately respond to it. In this regard, it remains unknown as to whether SCs play the role of a passive bystander or an active director during the execution of the highly orchestrated disintegration program of axons. Older reports, together with more recent studies, suggest that SCs mount dynamic injury responses minutes after axonal injury, long before axonal breakdown occurs. The swift SC response to axonal injury could play either a pro-degenerative role, or alternatively a supportive role, to the integrity of distressed axons that have not yet committed to degenerate. Indeed, supporting the latter concept, recent findings in a chronic PNS neurodegeneration model indicate that deactivation of a key molecule promoting SC injury responses exacerbates axonal loss. If this holds true in a broader spectrum of conditions, it may provide the grounds for the development of new glia-centric therapeutic approaches to counteract axonal loss.
Keywords: Wallerian degeneration, neurodegeneration, glia, oligodendrocytes, myelin, dedifferentiation
Introduction: Degeneration of the Axon-Glia Unit in Disease and after Injury
Axons are the thin, often meter-long projections of neurons which electrically wire the nervous system. These cable-like structures are interlaced with glial cells they closely interact with to establish a unique bidirectional relationship (Nave and Trapp, 2008; Beirowski, 2013). In the peripheral nervous system (PNS) of vertebrate species axons are associated with Schwann cells (SCs), while brain and spinal cord axons in the central nervous system (CNS) are surrounded by oligodendrocytes (OLGs). Under basal conditions these very specialized glial cells regulate the axonal cytoskeletal composition and ion channel distribution, provide support for axons, and often form compact myelin sheaths around axons, which provide faster electrical signal propagation. Because of their enormous length and extraordinary energetic demands (the axonal volume roughly exceeds the neuronal cell body volume by 1,000-fold), axons and their glia are particularly vulnerable structures and are at continuous risk of damage. Thus it is not surprising that axonal degeneration, along with alterations in their glia, is a hallmark of a wide range of neurodegenerative conditions that cause substantial morbidity and socioeconomic burden to society (Coleman and Perry, 2002; Raff et al., 2002). Examples include Alzheimer's and Parkinsons diseases, as well as many hereditary and acquired neuropathies such as diabetic neuropathy (Cashman and Hoke, 2015; Feldman et al., 2017). Importantly, axonal degeneration is not merely a pathological epiphenomenon that accompanies neuronal demise or a late-stage consequence of neuron cell body death. Instead, axonal degeneration is an early event in neurodegeneration, and a major cause of irreversible neurological disability in several of the aforementioned diseases. The etiology of axonal death and whether it is caused by cell autonomous or non-cell autonomous mechanisms in these conditions is poorly understood. The possible contribution of glial effects for the progression of axonal degeneration in many neurodegenerative diseases is often neglected and overlooked. Instead, glial contributions to axonal degeneration are only explored when there exists direct evidence for axonal damage through toxic events in glia or neuroinflammation secondary to demyelination. Nonetheless, there is emerging evidence that pathways in SCs and OLGs may play a more instructive role, thus regulating the rate and degree of axonal degeneration, and perhaps even directly executing aspects of axonal breakdown. For example, in amyotrophic lateral sclerosis (ALS), a fatal neurodegenerative disease of spinal cord motor neurons characterized by early degeneration of their axonal projections, it appears that pathological events restricted to SCs and OLGs substantially impact axonal degeneration and overall disease progression (Lobsiger et al., 2009; Ferraiuolo et al., 2017). Thus, if SCs and OLGs play an active role during axonal degeneration, then it may be possible to stabilize axons by glial manipulations, thereby establishing novel therapeutic strategies. Indeed, we have recently shown that metabolic pathways in SCs controlled by the metabolic master regulator LKB1 (also known as Stk11) play an essential role for the maintenance of long axons (Beirowski et al., 2014). In mouse models with SCs lacking LKB1, axons degenerate as a consequence of glial metabolic abnormalities including abnormal bioenergetics. Our data indicated the glial release of lactate, recently proposed to be the mainstay metabolite for axonal support by OLGs (Funfschilling et al., 2012; Lee et al., 2012), is unlikely compromised in LKB1-deficient SCs (Beirowski et al., 2014). Rather, LKB1-deficient SCs seem to mount canonical SC-injury responses (see below) and respond with elevated lactate release to support metabolically distressed axons deprived of other critical components (Beirowski et al., 2014).
In this brief review, we will summarize what is currently known regarding SC behavior during axonal degeneration, and its relevance for axonal loss in disease. We will provide circumstantial evidence suggesting that SCs are active participants in the degenerative mechanisms that execute axonal dismantling in injured nerves. We will conclude by elaborating on the cellular and molecular mechanisms known to regulate the phenotypic conversion of SCs to a dedifferentiated state that confers neuroprotective and reparative functions in injured peripheral nerves.
SCs are the First Responders to Axonal Injury
How do SCs react and initially respond to axonal injury that eventually may lead to axonal loss? Because the nature of axonal injury in many neurodegenerative conditions remains elusive, and axonal dismantling in chronic neurodegenerative conditions is a rather slow, asynchronous, and stochastic process, models of experimentally-induced acute axonal degeneration are better suited to explore this question. Wallerian degeneration (WD) is a straightforward experimental model system used to study the fast and simultaneously-induced degeneration of all axons within a nerve (Waller, 1850). In rodents, axonal breakdown during WD occurs within the span of few days in the distal stump of a transected nerve after all axons are separated from their parent neuronal cell bodies (e.g., after surgical transection of the rodent sciatic nerve) (Beirowski et al., 2004). The concomitant alterations in non-neuronal components during WD include prominent reactive injury responses of SCs that eventually lead to the disassembly and digestion of the myelin sheaths (referred to as myelin ovoid formation), breach of the blood-nerve barrier, macrophage recruitment, and a complex process of nerve tissue remodeling in preparation for nerve repair (Cattin and Lloyd, 2016) (Figure 1). An intricate network of transcriptional changes in the distal nerve stump is associated with these reactive SC changes (Yi et al., 2015). The disintegration of axons itself is characterized by a heterogeneous latency phase (about 1 day in mouse sciatic nerve) in which individual transected axons first appear grossly normal but then, roughly within an hour, abruptly undergo a catastrophic fragmentation process with morphological similarities to programmed cell death (Beirowski et al., 2005). It is now recognized that this sudden self-destruction of axons after a period of latency, known as the commitment phase of WD, is actively regulated by molecular mechanisms in neurons that are distinct from canonical cell death pathways such as apoptosis (Wang et al., 2012; Gerdts et al., 2016). This pathway can be potently blocked genetically by the overexpression of negative regulators (i.e., Wallerian degeneration slow protein (WldS) and its variants (Mack et al., 2001; Beirowski et al., 2009, 2010; Babetto et al., 2010)), or the deactivation of positive regulators of WD (i.e., SARM1 (Sterile Alpha and TIR Motif 1), Phr1 (PAM-highwire-Rpm-1), TMEM184b, death receptor 6 (DR6) (Osterloh et al., 2012; Babetto et al., 2013; Bhattacharya et al., 2016; Gamage et al., 2017)), resulting in remarkable protection of transected axons in mutant mice. In contrast, in wild-type animals, the engagement of this degenerative pathway results in a rapid depletion of NAD+ and ATP, a rise of intra-axonal calcium levels, and an activation of so-called calpains and other downstream molecules leading to the swift enzymatic digestion of the axonal cytoskeleton. Strikingly, a number of reactive SC changes have been documented in WD markedly preceding this disintegration phase of axons (i.e., occurring within the latency phase), indicating that SCs ‘sense’ axonal injury long distances away from the lesion point along axon portions that do not show morphological evidence of degeneration. This temporal and topological pattern is consistent with a model suggesting that SCs could regulate the commitment of axons to disintegrate. In addition, SCs could also dictate the onset and timing of axon fragmentation once this commitment has been established. In fact, reports from more than five decades ago demonstrate the retraction of paranodal SC components, a corresponding widening of the node of Ranvier dividing two adjacent SC myelin sheaths, and a prominent dilation of Schmidt-Lanterman incisures (SLI) that occurs within minutes to a few hours after a nerve lesion (Causey and Palmer, 1953; Williams and Hall, 1971; Ghabriel and Allt, 1979a, b). SLI are thin SC cytoplasmic channels within compact myelin of the fiber's internode, denoting the SC body area that overlies and enwraps axonal segments with myelin. Paranodes are other areas of direct axon-glia contact containing paranodal junctions where close apposition between SC and axon cytoplasm can be found. Alterations at paranodes, nodes, and SLI, together with further dynamic changes in these regions were interpreted by some authors as indicative for an active role of the SC in the breakdown of the axon (Singer and Steinberg, 1972). Notably, it has long since been speculated that the cytoplasmic channels within SCs serve as routes for trophic support of axons (Williams and Landon, 1963), suggesting the idea that SCs respond to axonal injury by augmenting trophic communication routes between the axonal and the glial cytoplasm. Alternatively, it is possible that the observed alterations allow the transfer of deleterious enzymes harmful to axons that may initiate axonal disintegration (De et al., 2003). Another deleterious substrate in such cytoplasmic channels could be calcium, which has been shown in numerous studies to be a downstream executioner of axonal disintegration (Avery et al., 2012; Yang et al., 2013; Villegas et al., 2014). In fact, axoplasmic calcium elevations at nodes of Ranvier have been observed after paranodal myelin disruption (Zhang and David, 2016), and another recent study using genetically encoded calcium sensors and multiphoton microscopy suggested an early and dynamic mitochondria-derived calcium elevation in the SC cytoplasm, starting about one hour after peripheral nerve injury (Gonzalez et al., 2016a, b, c) [note that this publication has been retracted during the review of this manuscript on grounds of errors in presentation of data other than the proposed calcium release after nerve injury (Gonzalez et al., 2017)].
Figure 1.
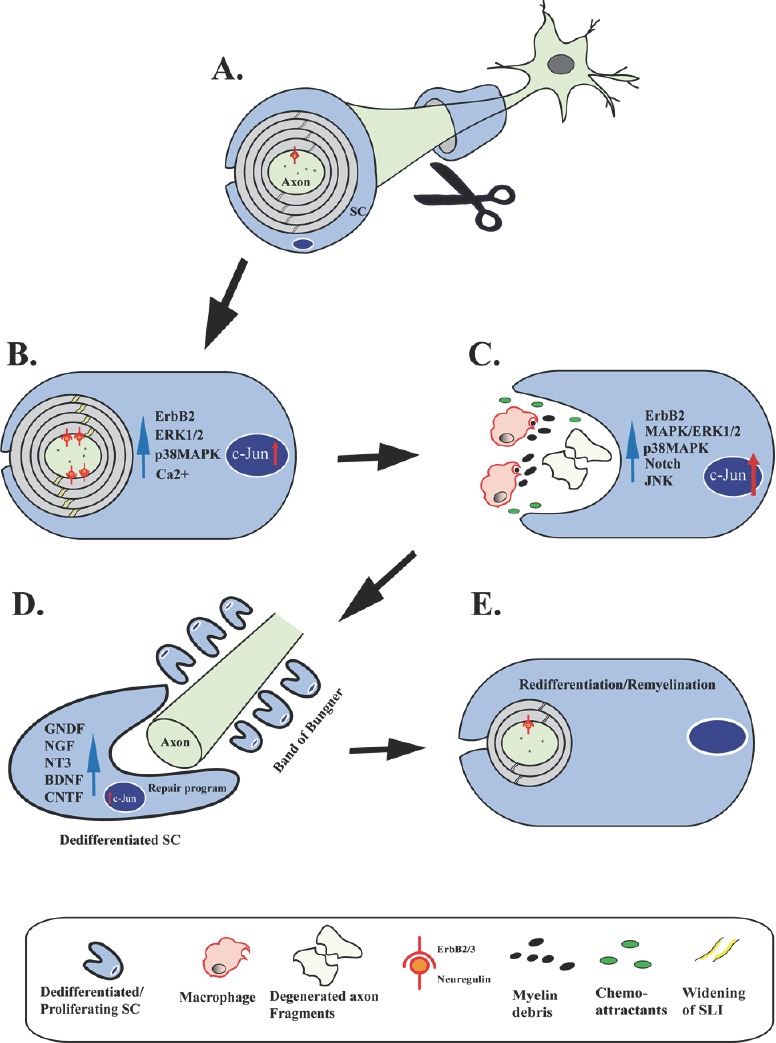
Schwann cell (SC) responses following axotomy.
Schematic illustrating distinct stages of SC responses following axotomy and during axonal regeneration and remyelination. (A) Under basal conditions the axon is encapsulated by a compact myelin sheath, established by Neuregulin-ErbB2/3 signaling during development. (B) Upon injury the first reactions in the SC, already minutes to 1 hour after axotomy, include widening of Schmidt-Lanterman incisures (yellow stripes in compact myelin), activation of the ErbB2 receptor tyrosine kinase (red anchors), activation of p38- and Erk1/2 mitogen-activated protein kinase (MAPK) signaling, and rapid increase of cytoplasmic calcium levels in the SC. Increased expression of c-Jun is also observed few hours after nerve injury. Actin progressively polymerizes in widened Schmidt-Lanterman incisures (SLI) (not depicted). These changes are accompanied by a general increase of the SC body size. (C) Following a latency phase with axons showing no morphological evidence of degeneration, axons then abruptly disintegrate leaving axon fragments behind. In parallel, during Wallerian degeneration (WD) there is formation of myelin debris secondary to rapid disassembly of the myelin sheaths. SCs release cytokines and chemokines that attract macrophages. In addition, SCs robustly upregulate injury response pathways including MAPK kinase pathways, Notch signaling, as well as further expression increases of c-Jun. (D) Axonal regeneration following WD is underway with SCs forming bands of Bungner and releasing surface molecules (not shown) and a multitude of neurotrophic factors including glial cell-derived neurotrophic factor (GDNF), nerve growth factor (NGF), neurotrophin-3 (NT3), brain-derived neurotrophic factor (BDNF), and ciliary neurotrophic factor (CNTF). This release is regulated by c-Jun in the SC. (E) SCs eventually conclude their repair program and redifferentiate to promote completion of nerve repair. Regenerating axons are remyelinated by SCs to restore normal nerve function.
What are the alterations in regions of axon-glia contact on molecular level? Remarkably, SCs even centimeters away from the nerve lesion site respond within a few minutes to axonal injury by activation of the ErbB2 receptor tyrosine kinase. This change is accompanied by increased downstream p38 and Erk1/2 mitogen-activated protein kinase (MAPK) signaling, most prominently located at their paranodal microvilli (Guertin et al., 2005; Yang et al., 2012). During nerve development, this receptor heterodimerizes with the ErbB3 pseudo-kinase and can be activated by axonal neuregulin to regulate SC differentiation and myelination. However, because addition of recombinant neuregulin induces myelin breakdown in in vitro models of myelination (Zanazzi et al., 2001; Harrisingh et al., 2004), it is tempting to speculate that elevated axonal neuregulin ligands, presumably as a consequence of injury-induced axolemmal cleavage, provide a critical injury signal that is communicated to the SC. Notably, inhibition of such ErbB2 activation through pharmacological approaches suppresses the SC injury response and subsequent myelin breakdown both in vitro and in vivo (Guertin et al., 2005). In accord with blocked axon-glia communication following nerve lesion, ablation of axonal neuregulin in adult mice leads to attenuated nerve regeneration after nerve lesion (Fricker et al., 2011). Shortly after the occurrence of the described ErbB2 activation (~6 hours), actin in non-compact myelin areas polymerizes as assessed by F-actin labeling, which has been proposed as a molecular mechanism initializing myelin fragmentation during WD (i.e. ‘myelin ovoid’ formation) (Jung et al., 2011). As an alternative to axonal neuregulin ligand signaling, it is possible that release of damage-associated molecular patterns (DAMPs) or so-called alarmins from mitochondria of injured axons including mtDNA, Cytochrome C (Cyt c) and H2O2 contributes to SC injury responses because such substances lead to sustained activation of Erk MAPK signaling in SCs within minutes of exposure (Duregotti et al., 2015). It will be interesting in future studies to explore whether or not such early molecular reactions in SCs that ultimately lead to myelin dismantling also occur in paradigms of blocked axon disintegration following nerve injury (see above). Interestingly in this context, it has been recently proposed that the deterioration of axons and SC myelin after nerve injury can be dissociated from each other (Gamage et al., 2017).
Taken together, in addition to the established function that SCs actively disassemble myelin sheaths during WD, these earlier and more recent data suggest the concept that SCs may also actively participate in the degenerative signaling events that lead to axonal breakdown in injured nerves. This clearly contrasts with a model for a more passive role of these glia in this initiating phase of axonal degeneration with more active functions to follow later (Stoll and Muller, 1999; Hirata and Kawabuchi, 2002; Vargas and Barres, 2007; Jessen and Mirsky, 2008).
SCs Dedifferentiate after Nerve Injury to Evoke a Glial Nerve Repair Program
At roughly the same time that axons begin to undergo cytoskeletal disintegration and fragmentation during WD, SCs activate a dedifferentiation program that leads to a dramatic downregulation of myelin-synthesis genes in SCs. These genes encode the transcription factor Egr2, myelin basic protein (MBP), and P0 glycoprotein (P0) among many other myelin proteins (Jessen and Mirsky, 2008). In parallel, molecular markers characteristic of immature SCs such as the p75 neurotrophic receptor (p75NTR) and the glial fibrillary acidic protein (GFAP) are progressively upregulated. This is accompanied by various adaptive responses in SCs promoting lysosomal/autophagic digestion of axonal and myelin debris (Gomez-Sanchez et al., 2015; Jang et al., 2016), and re-entry of SCs into the cell cycle (Yang et al., 2008). SCs are also known to regulate the immune response of WD that leads to macrophage recruitment through the glial release of various chemoattractants such as tumor necrosis factor-α (TNF-α), chemoattractant protein-1 (MCP-1), and placental growth factor (Martini et al., 2008; Chaballe et al., 2011; Gaudet et al., 2011). This response seems to be counterbalanced by parallel release of anti-inflammatory molecules such as erythropoietin (Epo) and upregulation of its receptor in SCs to prevent excessive inflammation (Campana et al., 2006). The intricate phenotypic conversion of SCs, mostly referred to as dedifferentiation, is essential for the remarkable regeneration potential of PNS axons. Here, axonal regrowth is to large extent stimulated by release of a plethora of SC-derived neurotrophic factors and surface proteins, as well as the fact that SCs play a key role in the formation of specialized axon-growth guidance tracks, broadly known as bands of Bungner (Stoll and Muller, 1999) (Figure 1). Recent intriguing in vivo studies have demonstrated that the interactions of fibroblasts with SCs and the behavior of macrophages and endothelia at the nerve injury site play a critical role for successful axon regeneration and nerve repair (Parrinello et al., 2010; Cattin et al., 2015). Upon nerve transection, fibroblasts and SCs sort at the injury site via ephrin-B/EphB2 signaling interactions to form cords of SCs that subsequently guide regrowing axons (Parrinello et al., 2010). Prior to that macrophage-induced blood vessels escort these SC cords across the transection site (Cattin et al., 2015). Moreover, SCs release exosomes that are internalized by axons and aid axonal regrowth through stimulation of axonal growth cone dynamics (Lopez-Verrilli et al., 2013). These microvesicles of 50-100 nm in diameter contain RNAs and proteins such as the p75NTR and likely other molecules promoting axonal regeneration (Lopez-Verrilli and Court, 2012). Eventually, once axonal elongation and target-reinnervation occurs, SCs gradually once again adopt their differentiated cellular state to support functions such as myelination of large-diameter axons and normal engulfment of small-diameter fibers in Remak bundles (i.e., SCs encapsulating bundles of several small-diameter unmyelinated axons). These phenotypic switches as a reaction to axonal injury, and later repair, reflect the extraordinary plasticity of SC glia. Such plastic behavior is lacking in OLGs, the myelinating glia counterpart in the CNS, perhaps directly causing the protracted WD in the CNS (Figure 2). Thus OLGs do not promote axon regrowth, resulting in the poor regeneration capacity of the CNS (Vargas and Barres, 2007). Notably, and similar in principle, in circumstances of genetically blocked axonal self-destruction (impressively seen in the so-called WldS mouse), SCs do not dedifferentiate which results in compromised axonal regeneration (Brown et al., 1992; Arthur-Farraj et al., 2012).
Figure 2.
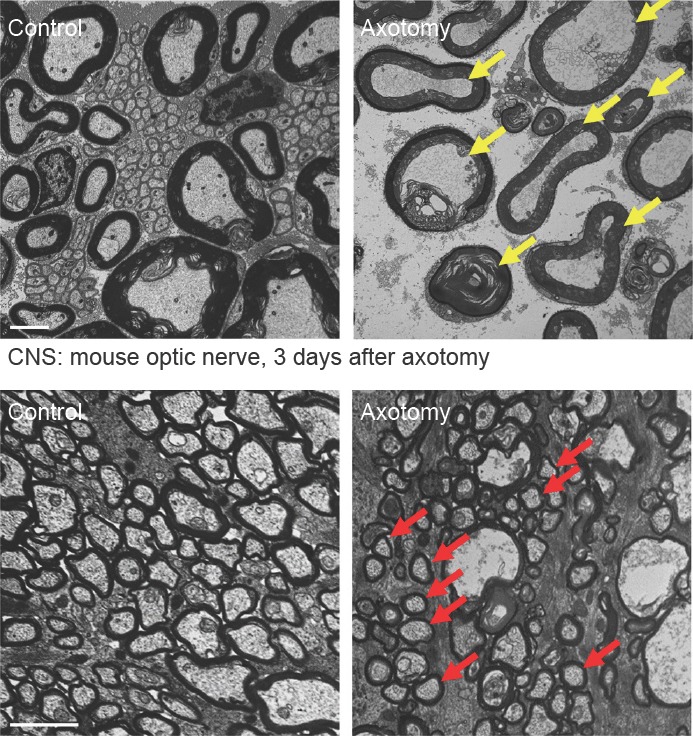
Wallerian degeneration is protracted in the central nervous system.
Transmission electron microscopy of control and axotomized transverse mouse sciatic nerves (upper row) and optic nerves (lower row) representing Wallerian degeneration of the peripheral nervous system (PNS) and central nervous system (CNS), respectively. Note complete degeneration of all PNS axons with granular disintegration of the axoplasm in the distal nerve stump three days following axotomy (yellow arrows depict examples). In contrast, many CNS axons are structurally preserved three days following optic nerve axotomy (red arrows depict examples). This indicates that Wallerian degeneration of axons progresses slower in the CNS than in the PNS. Scale bars: 2 μm.
Based on detailed in vitro and in vivo studies, it has been increasingly appreciated in recent years that the WD-associated reprogramming from differentiated SCs to their repair phenotype is regulated by a molecular cascade centered around the AP-1 transcription factor c-Jun and a number of signaling pathways including Ras/Raf/MEK/ERK, p38-MAPK, JUN N-terminal kinase (JNK)-MAPK, and Notch (Agthong et al., 2006; Parkinson et al., 2008; Woodhoo et al., 2009; Monje et al., 2010; Arthur-Farraj et al., 2012; Napoli et al., 2012; Yang et al., 2012) (Figure 1). The expression of c-Jun after nerve injury and activation of above signaling pathways can be prominently manipulated by the tumor suppressor Merlin and downstream Hippo pathway effector YAP (Mindos et al., 2017). Also very recently, it has been additionally shown that the transcription factor STAT3 plays a role in sustaining the c-Jun dependent SC repair phenotype by promoting long-term SC survival and expression of trophic factors important for axonal regeneration following axon injury (Benito et al., 2017). Moreover, epigenetic polycomb silencing regulating repressive histone modifications (methylation) in SCs after nerve injury has been implicated in the control of c-Jun dependent SC repair genes (Ma et al., 2016). How exactly all these factors work together to coordinate the complex program of SC dedifferentiation, and whether they converge on a common pathway, is essentially unknown so far. After nerve injury, above signaling components are promptly activated in SCs, and some have been documented separately as negative regulators of myelin genes (Parkinson et al., 2008; Woodhoo et al., 2009; Yang et al., 2012). It is interesting to note that the upregulation of c-Jun expression in SCs can be observed within a few hours of nerve injury, suggesting further direct molecular links between the SC dedifferentiation pathways and the earliest SC responses described above. For example, it is known that activation of JNK-MAPK signaling is a key enhancer of c-Jun activity, and there is considerable crosstalk between JNK-MAPK signaling and other branches of MAPK signaling (Monje et al., 2010). The induction of the extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) has been shown to promote the inflammatory responses of dedifferentiated SCs such as release of the chemoattractant MCP-1 that is necessary to entice macrophages to injured nerves (Napoli et al., 2012). Importantly, the genetic inactivation of c-Jun in SCs results in dramatic axonal regeneration defects and neuronal demise in vivo secondary to the shutdown of various SC dedifferentiation responses including abolished suppression of myelin clearance and release of neurotrophic factors from SCs (Parkinson et al., 2008; Arthur-Farraj et al., 2012; Fontana et al., 2012). Intriguingly, it has been demonstrated in vivo that the SC dedifferentiation response can be activated in the complete absence of WD (through genetic induction of Ras/Raf/MEK/ERK or Notch signaling in SCs), thus decoupling the WD-associated SC responses from axon injury (Woodhoo et al., 2009; Napoli et al., 2012). It is unknown if the artificial activation of the other pathways (i.e., JNK-MAPK, p38-MAPK) implicated in SC dedifferentiation may result in similar effects. Consistent with a hierarchy for these pathways regulating SC dedifferentiation, it appears that p38-MAPK signaling has a less important role because loss of p38-MAPK activity in SCs has no impact on axonal regeneration and functional nerve repair in mutant mice, and myelin clearance during WD is only slightly delayed (Roberts et al., 2017). The injury-induced dedifferentiation program of SCs can also be influenced by the ubiquitin-proteasome system (UPS) (Lee et al., 2009), presumably because of the possible involvement of the UPS in the degradation of myelin components. On the other hand, both in vitro and in vivo studies showed that proteasome inhibition prevents SCs from upregulating dedifferentiation markers such as p75NTR and GFAP upon injury (Lee et al., 2009). Furthermore, it is possible that SC dedifferentiation and demyelination is regulated by the earlier-mentioned rapid calcium burst in SCs undergoing WD (Gonzalez et al., 2016a, b, c) [note that this publication has been retracted during the review of this manuscript on grounds of errors in presentation of data other than the proposed calcium release after nerve injury (Gonzalez et al., 2017)]. Hence, because cytoplasmic calcium is known to induce the activation of several of these signaling pathways, it is conceivable that this mechanism represents the initiating event in the complex SC dedifferentiation cascade. Finally, this mechanism establishes also intriguing links between metabolic neuropathies (e.g., diabetic neuropathy) that are clearly associated with mitochondrial abnormalities, SC dedifferentiation, demyelination of nerves, as well as axonal degeneration (Fernyhough and Calcutt, 2010; Chowdhury et al., 2013; Zenker et al., 2013; Gonzalez et al., 2016b; Feldman et al., 2017).
SC Injury Responses Limit Axonal Loss in Disease
Since induction of c-Jun and Erk1/2 signaling in SCs also occurs in neuropathic disease contexts in which axons die slowly (Hutton et al., 2011; Klein et al., 2014), a critical question is whether such upregulation is part of an endogenous neuroprotective mechanism to support distressed axons. We also observed substantial upregulation of c-Jun expression in LKB1-deficient SCs that cause an age-dependent and progressive axonopathy, and in parallel elicit compensatory axon-supportive features (Beirowski et al., 2014). Remarkably, a recent study has shown that elimination of the increased c-Jun expression in SCs in a Charcot-Marie-Tooth (CMT) 1A neuropathy model results in marked accentuation of axonal loss and deterioration in sensory-motor performance (Hantke et al., 2014). It will be important to explore the mechanistic basis of this finding in the future. For example, it is essential to investigate whether the prevention of c-Jun upregulation abolishes the increased release of trophic factors or other neuroprotective responses in the mutant SCs which could directly promote axonal degeneration. Alternatively, reduced levels of c-Jun induction in SCs have been recently implicated in the age-related decline of PNS axonal regeneration (Painter et al., 2014), and the reduced axon numbers in above neuropathy model therefore could be secondary to diminished axonal regeneration.
Conclusions and Perspectives
In this article, we presented evidence that SCs mount sophisticated responses rapidly following axonal injury through poorly understood molecular and cellular mechanisms. It is essentially unknown if these reactions are maladaptive or neuroprotective or neutral. These responses culminate in the induction of a complex glial injury program that converts SCs to dedifferentiated cells that promote axonal regeneration and peripheral nerve repair. Future studies will be needed to explore whether the augmentation of different aspects of the SC injury response program has neuroprotective potential for a range of diseases with degeneration of axons as a major etiological component.
Acknowledgments
We are grateful to Nadav Weinstock for careful reading of the manuscript and helpful comments. We apologize to colleagues whose work was not cited because of space constraints.
Footnotes
Funding: The work in our laboratory is supported by Muscular Dystrophy Association grants #292306 and #236648, Empire State Development Corporation for HJKRI Grants W753 and U446, Hunter's Hope Foundation, and University at Buffalo IMPACT funding.
References
- Agthong S, Kaewsema A, Tanomsridejchai N, Chentanez V. Activation of MAPK ERK in peripheral nerve after injury. BMC Neurosci. 2006;7:45. doi: 10.1186/1471-2202-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery MA, Rooney TM, Pandya JD, Wishart TM, Gillingwater TH, Geddes JW, Sullivan PG, Freeman MR. WldS prevents axon degeneration through increased mitochondrial flux and enhanced mitochondrial Ca2+ buffering. Curr Biol. 2012;22:596–600. doi: 10.1016/j.cub.2012.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babetto E, Beirowski B, Russler EV, Milbrandt J, DiAntonio A. The Phr1 ubiquitin ligase promotes injury-induced axon self-destruction. Cell Rep. 2013;3:1422–1429. doi: 10.1016/j.celrep.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babetto E, Beirowski B, Janeckova L, Brown R, Gilley J, Thomson D, Ribchester RR, Coleman MP. Targeting NMNAT1 to axons and synapses transforms its neuroprotective potency in vivo. J Neurosci. 2010;30:13291–13304. doi: 10.1523/JNEUROSCI.1189-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B. Concepts for regulation of axon integrity by enwrapping glia. Frontiers Cell Neurosci. 2013;7:256. doi: 10.3389/fncel.2013.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B, Adalbert R, Wagner D, Grumme DS, Addicks K, Ribchester RR, Coleman MP. The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves. BMC Neurosci. 2005;6:6. doi: 10.1186/1471-2202-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B, Berek L, Adalbert R, Wagner D, Grumme DS, Addicks K, Ribchester RR, Coleman MP. Quantitative and qualitative analysis of Wallerian degeneration using restricted axonal labelling in YFP-H mice. J Neurosci Methods. 2004;134:23–35. doi: 10.1016/j.jneumeth.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Beirowski B, Babetto E, Golden JP, Chen YJ, Yang K, Gross RW, Patti GJ, Milbrandt J. Metabolic regulator LKB1 is crucial for Schwann cell-mediated axon maintenance. Nat Neurosci. 2014;17:1351–1361. doi: 10.1038/nn.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B, Babetto E, Gilley J, Mazzola F, Conforti L, Janeckova L, Magni G, Ribchester RR, Coleman MP. Non-nuclear Wld(S) determines its neuroprotective efficacy for axons and synapses in vivo. J Neurosci. 2009;29:653–668. doi: 10.1523/JNEUROSCI.3814-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B, Morreale G, Conforti L, Mazzola F, Di Stefano M, Wilbrey A, Babetto E, Janeckova L, Magni G, Coleman MP. WldS can delay Wallerian degeneration in mice when interaction with valosin-containing protein is weakened. Neuroscience. 2010;166:201–211. doi: 10.1016/j.neuroscience.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Benito C, Davis CM, Gomez-Sanchez JA, Turmaine M, Meijer D, Poli V, Mirsky R, Jessen KR. STAT3 controls the long-term survival and phenotype of repair Schwann cells during nerve regeneration. J Neurosci. 2017 doi: 10.1523/JNEUROSCI.3481-16.2017. doi:10.1523/JNEUROSCI.3481-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya MR, Geisler S, Pittman SK, Doan RA, Weihl CC, Milbrandt J, DiAntonio A. TMEM184b Promotes axon degeneration and neuromuscular junction maintenance. J Neurosci. 2016;36:4681–4689. doi: 10.1523/JNEUROSCI.2893-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Lunn ER, Perry VH. Consequences of slow Wallerian degeneration for regenerating motor and sensory axons. J Neurobiol. 1992;23:521–536. doi: 10.1002/neu.480230507. [DOI] [PubMed] [Google Scholar]
- Campana WM, Li X, Shubayev VI, Angert M, Cai K, Myers RR. Erythropoietin reduces Schwann cell TNF-alpha, Wallerian degeneration and pain-related behaviors after peripheral nerve injury. Eur J Neurosci. 2006;23:617–626. doi: 10.1111/j.1460-9568.2006.04606.x. [DOI] [PubMed] [Google Scholar]
- Cashman CR, Hoke A. Mechanisms of distal axonal degeneration in peripheral neuropathies. Neurosci Lett. 2015;596:33–50. doi: 10.1016/j.neulet.2015.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattin AL, Lloyd AC. The multicellular complexity of peripheral nerve regeneration. Curr Opin Neurobiol. 2016;39:38–46. doi: 10.1016/j.conb.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Cattin AL, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJ, Garcia Calavia N, Guo Y, McLaughlin M, Rosenberg LH, Quereda V, Jamecna D, Napoli I, Parrinello S, Enver T, Ruhrberg C, Lloyd AC. Macrophage-induced blood vessels guide schwann cell-mediated regeneration of peripheral nerves. Cell. 2015;162:1127–1139. doi: 10.1016/j.cell.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causey G, Palmer E. The centrifugal spread of structural change at the nodes in degenerating mammalian nerves. J Anat. 1953;87:185–191. [PMC free article] [PubMed] [Google Scholar]
- Chaballe L, Close P, Sempels M, Delstanche S, Fanielle J, Moons L, Carmeliet P, Schoenen J, Chariot A, Franzen R. Involvement of placental growth factor in Wallerian degeneration. Glia. 2011;59:379–396. doi: 10.1002/glia.21108. [DOI] [PubMed] [Google Scholar]
- Chowdhury SK, Smith DR, Fernyhough P. The role of aberrant mitochondrial bioenergetics in diabetic neuropathy. Neurobiol Dis. 2013;51:56–65. doi: 10.1016/j.nbd.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Perry VH. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci. 2002;25:532–537. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- De S, Trigueros MA, Kalyvas A, David S. Phospholipase A2 plays an important role in myelin breakdown and phagocytosis during Wallerian degeneration. Mol Cell Neurosci. 2003;24:753–765. doi: 10.1016/s1044-7431(03)00241-0. [DOI] [PubMed] [Google Scholar]
- Duregotti E, Negro S, Scorzeto M, Zornetta I, Dickinson BC, Chang CJ, Montecucco C, Rigoni M. Mitochondrial alarmins released by degenerating motor axon terminals activate perisynaptic Schwann cells. Proc Natl Acad Sci U S A. 2015;112:E497–505. doi: 10.1073/pnas.1417108112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman EL, Nave KA, Jensen TS, Bennett DL. New horizons in diabetic neuropathy: Mechanisms, bioenergetics, and pain. Neuron. 2017;93:1296–1313. doi: 10.1016/j.neuron.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernyhough P, Calcutt NA. Abnormal calcium homeostasis in peripheral neuropathies. Cell Calcium. 2010;47:130–139. doi: 10.1016/j.ceca.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraiuolo L, Meyer K, Sherwood TW, Vick J, Likhite S, Frakes A, Miranda CJ, Braun L, Heath PR, Pineda R, Beattie CE, Shaw PJ, Askwith CC, McTigue D, Kaspar BK. Oligodendrocytes contribute to motor neuron death in ALS via SOD1-dependent mechanism. Proc Natl Acad Sci U S A. 2017;113:E6496–6505. doi: 10.1073/pnas.1607496113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana X, Hristova M, Da Costa C, Patodia S, Thei L, Makwana M, Spencer-Dene B, Latouche M, Mirsky R, Jessen KR, Klein R, Raivich G, Behrens A. c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. J Cell Biol. 2012;198:127–141. doi: 10.1083/jcb.201205025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker FR, Lago N, Balarajah S, Tsantoulas C, Tanna S, Zhu N, Fageiry SK, Jenkins M, Garratt AN, Birchmeier C, Bennett DL. Axonally derived neuregulin-1 is required for remyelination and regeneration after nerve injury in adulthood. J Neurosci. 2011;31:3225–3233. doi: 10.1523/JNEUROSCI.2568-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamage KK, Cheng I, Park RE, Karim MS, Edamura K, Hughes C, Spano AJ, Erisir A, Deppmann CD. Death receptor 6 promotes Wallerian degeneration in peripheral axons. Curr Biol. 2017;27:890–896. doi: 10.1016/j.cub.2017.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts J, Summers DW, Milbrandt J, DiAntonio A. Axon self-destruction: New links among SARM1, MAPKs, and NAD+ metabolism. Neuron. 2016;89:449–460. doi: 10.1016/j.neuron.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghabriel MN, Allt G. The role of Schmidt-Lanterman incisures in Wallerian degeneration. II. An electron microscopic study. Acta Neuropathol. 1979a;48:95–103. doi: 10.1007/BF00691150. [DOI] [PubMed] [Google Scholar]
- Ghabriel MN, Allt G. The role of Schmidt-Lanterman incisures in Wallerian degeneration. I. A quantitative teased fibre study. Acta Neuropathol (Berl) 1979b;48:93–93. [PubMed] [Google Scholar]
- Gomez-Sanchez JA, Carty L, Iruarrizaga-Lejarreta M, Palomo-Irigoyen M, Varela-Rey M, Griffith M, Hantke J, Macias-Camara N, Azkargorta M, Aurrekoetxea I, De Juan VG, Jefferies HB, Aspichueta P, Elortza F, Aransay AM, Martínez-Chantar ML, Baas F, Mato JM, Mirsky R, Woodhoo A, et al. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J Cell Biol. 2015;210:153–168. doi: 10.1083/jcb.201503019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Berthelot J, Jiner J, Perrin-Tricaud C, Fernando R, Chrast R, Lenaers G, Tricaud N. Blocking mitochondrial calcium release in Schwann cells prevents demyelinating neuropathies. J Clin Invest. 2016a;126:2773. doi: 10.1172/JCI88179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Berthelot J, Jiner J, Perrin-Tricaud C, Fernando R, Chrast R, Lenaers G, Tricaud N. Blocking mitochondrial calcium release in Schwann cells prevents demyelinating neuropathies. J Clin Invest. 2016b;126:1023–1038. doi: 10.1172/JCI84505. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gonzalez S, Berthelot J, Jiner J, Perrin-Tricaud C, Fernando R, Chrast R, Lenaers G, Tricaud N. Blocking mitochondrial calcium release in Schwann cells prevents demyelinating neuropathies. J Clin Invest. 2016c;126:1605. doi: 10.1172/JCI87203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Berthelot J, Jiner J, Perrin-Tricaud C, Fernando R, Chrast R, Lenaers G, Tricaud N. Blocking mitochondrial calcium release in Schwann cells prevents demyelinating neuropathies. J Clin Invest. 2017;127:1115. doi: 10.1172/JCI92100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin AD, Zhang DP, Mak KS, Alberta JA, Kim HA. Microanatomy of axon/glial signaling during Wallerian degeneration. J Neurosci. 2005;25:3478–3487. doi: 10.1523/JNEUROSCI.3766-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke J, Carty L, Wagstaff LJ, Turmaine M, Wilton DK, Quintes S, Koltzenburg M, Baas F, Mirsky R, Jessen KR. c-Jun activation in Schwann cells protects against loss of sensory axons in inherited neuropathy. Brain. 2014;137:2922–2937. doi: 10.1093/brain/awu257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrisingh MC, Perez-Nadales E, Parkinson DB, Malcolm DS, Mudge AW, Lloyd AC. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. Embo J. 2004;23:3061–3071. doi: 10.1038/sj.emboj.7600309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata K, Kawabuchi M. Myelin phagocytosis by macrophages and nonmacrophages during Wallerian degeneration. Microsc Res Tech. 2002;57:541–547. doi: 10.1002/jemt.10108. [DOI] [PubMed] [Google Scholar]
- Hutton EJ, Carty L, Laura M, Houlden H, Lunn MP, Brandner S, Mirsky R, Jessen K, Reilly MM. c-Jun expression in human neuropathies: a pilot study. J Peripher Nerv Syst. 2011;16:295–303. doi: 10.1111/j.1529-8027.2011.00360.x. [DOI] [PubMed] [Google Scholar]
- Jang SY, Shin YK, Park SY, Park JY, Lee HJ, Yoo YH, Kim JK, Park HT. Autophagic myelin destruction by Schwann cells during Wallerian degeneration and segmental demyelination. Glia. 2016;64:730–742. doi: 10.1002/glia.22957. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56:1552–1565. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- Jung J, Cai W, Lee HK, Pellegatta M, Shin YK, Jang SY, Suh DJ, Wrabetz L, Feltri ML, Park HT. Actin polymerization is essential for myelin sheath fragmentation during Wallerian degeneration. J Neurosci. 2011;31:2009–2015. doi: 10.1523/JNEUROSCI.4537-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D, Groh J, Wettmarshausen J, Martini R. Nonuniform molecular features of myelinating Schwann cells in models for CMT1: distinct disease patterns are associated with NCAM and c-Jun upregulation. Glia. 2014;62:736–750. doi: 10.1002/glia.22638. [DOI] [PubMed] [Google Scholar]
- Lee HK, Shin YK, Jung J, Seo SY, Baek SY, Park HT. Proteasome inhibition suppresses Schwann cell dedifferentiation in vitro and in vivo. Glia. 2009;57:1825–1834. doi: 10.1002/glia.20894. [DOI] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobsiger CS, Boillee S, McAlonis-Downes M, Khan AM, Feltri ML, Yamanaka K, Cleveland DW. Schwann cells expressing dismutase active mutant SOD1 unexpectedly slow disease progression in ALS mice. Proc Natl Acad Sci U S A. 2009;106:4465–4470. doi: 10.1073/pnas.0813339106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Verrilli MA, Court FA. Transfer of vesicles from schwann cells to axons: a novel mechanism of communication in the peripheral nervous system. Front Physiol. 2012;3:205. doi: 10.3389/fphys.2012.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013;61:1795–1806. doi: 10.1002/glia.22558. [DOI] [PubMed] [Google Scholar]
- Ma KH, Hung HA, Svaren J. Epigenomic regulation of Schwann cell reprogramming in peripheral nerve injury. J Neurosci. 2016;36:9135–9147. doi: 10.1523/JNEUROSCI.1370-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack TG, Reiner M, Beirowski B, Mi W, Emanuelli M, Wagner D, Thomson D, Gillingwater T, Court F, Conforti L, Fernando FS, Tarlton A, Andressen C, Addicks K, Magni G, Ribchester RR, Perry VH, Coleman MP. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci. 2001;4:1199–1206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- Martini R, Fischer S, Lopez-Vales R, David S. Interactions between Schwann cells and macrophages in injury and inherited demyelinating disease. Glia. 2008;56:1566–1577. doi: 10.1002/glia.20766. [DOI] [PubMed] [Google Scholar]
- Mindos T, Dun XP, North K, Doddrell RD, Schulz A, Edwards P, Russell J, Gray B, Roberts SL, Shivane A, Mortimer G, Pirie M, Zhang N, Pan D, Morrison H, Parkinson DB. Merlin controls the repair capacity of Schwann cells after injury by regulating Hippo/YAP activity. J Cell Biol. 2017;216:495–510. doi: 10.1083/jcb.201606052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje PV, Soto J, Bacallao K, Wood PM. Schwann cell dedifferentiation is independent of mitogenic signaling and uncoupled to proliferation: role of cAMP and JNK in the maintenance of the differentiated state. J Biol Chem. 2010;285:31024–31036. doi: 10.1074/jbc.M110.116970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I, Noon LA, Ribeiro S, Kerai AP, Parrinello S, Rosenberg LH, Collins MJ, Harrisingh MC, White IJ, Woodhoo A, Lloyd AC. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. 2012;73:729–742. doi: 10.1016/j.neuron.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci. 2008;31:535–561. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA, MacDonald JM, Ziegenfuss JS, Milde S, Hou YJ, Nathan C, Ding A, Brown RH, Jr, Conforti L, Coleman M, Tessier-Lavigne M, et al. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science. 2012;337:481–484. doi: 10.1126/science.1223899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter MW, Brosius Lutz A, Cheng YC, Latremoliere A, Duong K, Miller CM, Posada S, Cobos EJ, Zhang AX, Wagers AJ, Havton LA, Barres B, Omura T, Woolf CJ. Diminished Schwann cell repair responses underlie age-associated impaired axonal regeneration. Neuron. 2014;83:331–343. doi: 10.1016/j.neuron.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon LA, Woodhoo A, Lloyd AC, Feltri ML, Wrabetz L, Behrens A, Mirsky R, Jessen KR. c-Jun is a negative regulator of myelination. J Cell Biol. 2008;181:625–637. doi: 10.1083/jcb.200803013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrinello S, Napoli I, Ribeiro S, Digby PW, Fedorova M, Parkinson DB, Doddrell RD, Nakayama M, Adams RH, Lloyd AC. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell. 2010;143:145–155. doi: 10.1016/j.cell.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- Roberts SL, Dun XP, Dee G, Gray B, Mindos T, Parkinson DB. The role of p38alpha in Schwann cells in regulating peripheral nerve myelination and repair. J Neurochem. 2017;141:37–47. doi: 10.1111/jnc.13929. [DOI] [PubMed] [Google Scholar]
- Singer M, Steinberg MC. Wallerian degeneration: a reevaluation based on transected and colchicine-poisoned nerves in the Amphibian, Triturus. Am J Anat. 1972;133:51–83. doi: 10.1002/aja.1001330105. [DOI] [PubMed] [Google Scholar]
- Stoll G, Muller HW. Nerve injury, axonal degeneration and neural regeneration: basic insights. Brain Pathol. 1999;9:313–325. doi: 10.1111/j.1750-3639.1999.tb00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci. 2007;30:153–179. doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- Villegas R, Martinez NW, Lillo J, Pihan P, Hernandez D, Twiss JL, Court FA. Calcium release from intra-axonal endoplasmic reticulum leads to axon degeneration through mitochondrial dysfunction. J Neurosci. 2014;34:7179–7189. doi: 10.1523/JNEUROSCI.4784-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller A. Experiments on the section of glossopharyngeal and hypoglossal nerves of the frog and observations uf the alternatives produced thereby in the structure of their primitive fibres. Philos Trans R Soc Lond B Biol Sci. 1850;140:423–429. [Google Scholar]
- Wang JT, Medress ZA, Barres BA. Axon degeneration: Molecular mechanisms of a self-destruction pathway. J Cell Biol. 2012;196:7–18. doi: 10.1083/jcb.201108111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PL, Landon DN. Paranodal apparatus of peripheral myelinated nerve fibres of mammals. Nature. 1963;198:670–673. doi: 10.1038/198670a0. [DOI] [PubMed] [Google Scholar]
- Williams PL, Hall SM. Prolonged in vivo observations of normal peripheral nerve fibres and their acute reactions to crush and deliberate trauma. J Anat. 1971;108:397–408. [PMC free article] [PubMed] [Google Scholar]
- Woodhoo A, Alonso MB, Droggiti A, Turmaine M, D’Antonio M, Parkinson DB, Wilton DK, Al-Shawi R, Simons P, Shen J, Guillemot F, Radtke F, Meijer D, Feltri ML, Wrabetz L, Mirsky R, Jessen KR. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat Neurosci. 2009;12:839–847. doi: 10.1038/nn.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DP, Zhang DP, Mak KS, Bonder DE, Pomeroy SL, Kim HA. Schwann cell proliferation during Wallerian degeneration is not necessary for regeneration and remyelination of the peripheral nerves: axon-dependent removal of newly generated Schwann cells by apoptosis. Mol Cell Neurosci. 2008;38:80–88. doi: 10.1016/j.mcn.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DP, Kim J, Syed N, Tung YJ, Bhaskaran A, Mindos T, Mirsky R, Jessen KR, Maurel P, Parkinson DB, Kim HA. p38 MAPK activation promotes denervated Schwann cell phenotype and functions as a negative regulator of Schwann cell differentiation and myelination. J Neurosci. 2012;32:7158–7168. doi: 10.1523/JNEUROSCI.5812-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Weimer RM, Kallop D, Olsen O, Wu Z, Renier N, Uryu K, Tessier-Lavigne M. Regulation of axon degeneration after injury and in development by the endogenous calpain inhibitor calpastatin. Neuron. 2013;80:1175–1189. doi: 10.1016/j.neuron.2013.08.034. [DOI] [PubMed] [Google Scholar]
- Yi S, Zhang H, Gong L, Wu J, Zha G, Zhou S, Gu X, Yu B. Deep sequencing and bioinformatic analysis of lesioned sciatic nerves after crush injury. PLoS One. 2015;10:e0143491. doi: 10.1371/journal.pone.0143491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanazzi G, Einheber S, Westreich R, Hannocks MJ, Bedell-Hogan D, Marchionni MA, Salzer JL. Glial growth factor/neuregulin inhibits Schwann cell myelination and induces demyelination. J Cell Biol. 2001;152:1289–1299. doi: 10.1083/jcb.152.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenker J, Ziegler D, Chrast R. Novel pathogenic pathways in diabetic neuropathy. Trends Neurosci. 2013;36:439–449. doi: 10.1016/j.tins.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Zhang Z, David G. Stimulation-induced Ca(2+) influx at nodes of Ranvier in mouse peripheral motor axons. J Physiol. 2016;594:39–57. doi: 10.1113/JP271207. [DOI] [PMC free article] [PubMed] [Google Scholar]


