Abstract
Objective
To evaluate the clinical presentation of retinoblastoma in Alexandria, Egypt, correlate the timing of accurate diagnosis with the presence of advanced disease and identify causes of delayed presentation.
Methods
Retrospective noncomparative single institution study reviews demographic and clinical data of all new children with retinoblastoma presenting to Alexandria Main University ocular oncology clinic (OOC) from January 2012 to June 2014. Diagnosis time was from initial parental complaint to retinoblastoma diagnosis and referral time was from retinoblastoma diagnosis to presentation to the Alexandria OCC. Delayed Diagnosis and referral were counted if >2 weeks. Advanced presentation is defined as clinical TNMH (8th edition) staging of cT2 or cT3 (international intraocular retinoblastoma classification group D or E) in at least one eye or the presence of extra-ocular disease (cT4).
Results
Seventy eyes of 47 children were eligible: 52% unilateral, 7% with family history and 96% presented with leukocorea. Sixty-four percent of children had advanced intraocular disease and none had extra-ocular disease. Delayed presentation occurred in 58% of children and was significantly associated with advanced disease in both unilaterally and bilaterally affected children (p = 0.003, 0.002 respectively). The delay in diagnosis was more in unilateral cases while the delay in referral was more in bilateral cases. The main cause of delayed presentation in unilateral retinoblastoma was misdiagnosis (30%) while parental shopping for second medical opinion (30%) was the main cause in bilateral children.
Conclusions
Delayed diagnosis is a problem affecting retinoblastoma management. Better medical education and training, health education and earlier screening are recommended to achieve earlier diagnosis.
Keywords: Retinoblastoma, Developing country, Delayed diagnosis, Cancer, National
Introduction
Retinoblastoma, the most common intraocular malignancy in children, represents 4% of childhood cancer and 8000 new cases annually.1, 2, 3 It occurs due to a mutation affecting both copies of the RB1 gene (13q14). It may be heritable (germ-line mutation) presenting with bilateral and 15% of unilateral disease, or unilateral disease, 85% nonheritable,2, 4 The median age of diagnosis is 24 months in unilateral disease and 9–12 months in bilateral disease.5
The most commonly used classification and staging system has been the international intraocular retinoblastoma classification (IIRC) 1, 6 that describes intraocular retinoblastoma from favorable (IIRC group A) to unfavorable (IIRC group E) according to the anticipated prognostic response to chemoreduction and focal therapy. IIRC doesn’t categorize extra-ocular cases. The 8th edition TNM cancer staging describes the progression of retinoblastoma on anatomical basis, and adds heritability as an additional prognostic feature. Advanced intraocular retinoblastoma is IIRC groups D and E (TNM stage cT2 and T3).7, 8
In Alexandria, the pediatric ocular oncology clinic started late 2005, when the Ophthalmology Department acquired Pediatric retinal imaging (Retcam®) and indirect laser (810 nm). These tools improved documentation of patients at diagnosis and follow-up, and treatment by focal therapy. There are no formal data on clinical presentation of retinoblastoma prior to 2005. From 2005 to 2011, two cohorts9, 10 were studied focusing mainly on evaluation of treatment protocols without detailed description or analysis of clinical presentation in Alexandria. Socioeconomic factors were found to be impacted by treatment decisions decided based on clinical presentation.11
In the current study, we studied the clinical presentation of all retinoblastoma patients that were not treated before, who presented to our clinic. We hypothesized that there earlier diagnosis would be related to better health education about this disease in Alexandria.
Methods
This is a retrospective non-comparative single institution study. The study is in accordance with the Declaration of Helsinki guidelines and Ethics review board approval was obtained. All children with retinoblastoma that presented to the pediatric outpatient clinic of Alexandria Main University Hospital from January 2012 to June 2014 were reviewed. Only newly diagnosed patients without previous treatment were included. All children with retinoblastoma that were on either active treatment or follow-up from previous years, and children with retinoblastoma who were treated elsewhere and referred for second opinion, were excluded from data analysis.
Data collected included child’s sex, family history, time lapse since first complain, date of referral, age of presentation, manifesting symptom, laterality, clinical parameters as type of tumor (endophytic, exophytic or mixed), number and location of tumors, IIRC classification of eyes, the presence of exudative retinal detachment, macular and optic nerve involvement, and the presence of extraocular extension. For all cases, the TNM stage was retrospectively scored.
Diagnosis time was defined as the time from initial parental complaint to the diagnosis of retinoblastoma by the referring ophthalmologist. Delayed Diagnosis was defined as >2 weeks from initial parental complains. Referral time is the time from the diagnosis of retinoblastoma to the presentation to the Alexandria ocular oncology (AOO) clinic. Delayed referral was defined as >2 weeks from retinoblastoma diagnosis. Total delay was measured from time of initial parental complain to the time of the first examination at AOO clinic. If the child was diagnosed as retinoblastoma by AOO clinic, the referral time was considered zero. The causes of delay were listed if known. If the initial diagnosis was incorrect, this was marked as misdiagnosis. Advanced presentation was defined as TNM8 stage cT2, cT3 (IIRC group D or E) in at least one eye or the presence of extra-ocular disease (cT4).
Data were analyzed using IBM SPSS software package version 20.0. Qualitative data were described using number and percent. Quantitative data were described using range (minimum and maximum) mean, standard deviation and median. Comparison between different groups regarding categorical variables was tested using Chi-square test. When more than 20% of the cells have expected count less than 5, correction for chi-square was conducted using Monte Carlo correction. If it reveals normal data distribution, parametric tests were applied. If the data were abnormally distributed, nonparametric tests were used. Significance of the obtained results was judged at the 5% level.
Results
Demographic characteristics
Records of 94 children were reviewed and 47 children were eligible during the specified duration. Table 1 demonstrates the demographic characteristics of the study group. The median of age at presentation was 24 months (range; 2–49 months). The median age for bilateral cases was 11 months versus 25 months for unilateral children, a statistically significant difference (p = 0.03, t-test). Seventy eyes were involved at presentation, evenly distributed between the two eyes. One child developed second eye involvement after 14 months and the second eye was excluded from the analysis. Table 2 demonstrates the clinical appearance of all included eyes. Advanced intraocular disease cT2, cT3 (IIRC D and E) was found in 45/70 (64%) of eyes; 20/24 (83%) of unilateral children, 16/23 (69%) of bilateral children and 36/47 (77%) of all studied children. Table 3 demonstrates the staging of all eyes using IIRC6 and TNM8 staging. No cases with extra-ocular disease or systemic metastasis at presentation were encountered.
Table 1.
Demographic characteristic of the studied cohort.
| Number (n = 47) | % | ||
|---|---|---|---|
| Sex | |||
| Male | 25 | 53 | |
| Female | 22 | 47 | |
| Family history | 3 | 6 | |
| Main complain | |||
| Leukocorea | 45 | 96 | |
| Strabismus | 2 | 4 | |
| Laterality | |||
| Unilateral | 24 | 51 | |
| Bilateral | 23 | 49 | |
| Age (years) | |||
| <2 | 22 | 47 | |
| 2–4 | 25 | 53 | |
Table 2.
Clinical characteristics of the studied cohort.
| Number (n = 70) | % | |
|---|---|---|
| Cataract | 1 | 1 |
| Elevated IOP | 10 | 14 |
| NVG | 9 | 13 |
| Type of tumor | ||
| Endophytic | 35 | 50 |
| Exophytic | 16 | 23 |
| Mixed | 19 | 27 |
| Number of tumors | ||
| Single | 46 | 66 |
| Two | 3 | 4 |
| >2 tumors | 21 | 30 |
| Exudative retinal detachment | 32 | 46 |
| Macular involvement | 55 | 79 |
| Optic nerve encroachment | 35 | 50 |
| Tumor seeds (vitreous/subretinal) | 49 | 70 |
| IIRC | ||
| A | 1 | 1 |
| B | 14 | 20 |
| C | 10 | 14 |
| D | 36 | 51 |
| E | 9 | 13 |
| TNM | ||
| T1(T1a/T1b) | 15(4/11) | 21 |
| T2(T2a/T2b) | 46(6/40) | 66 |
| T3(T3a/T3b/T3c/T3d/T3e) | 9(0,0,9,0,0) | 13 |
| T4 | 0 | 0 |
Table 3.
Retinoblastoma staging using the international intraocular retinoblastoma classification (IIRC) and the TNM 8th edition staging in both eyes.
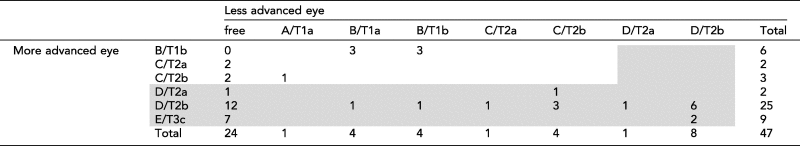 |
Advanced presentation is highlighted in grey.
Diagnosis delay
The median diagnosis time was 6 weeks (1–15 weeks) for unilateral patients and 3 weeks (1–6 weeks) for bilateral patients. The diagnosis time was significantly shorter for bilaterally affected children (Mood’s Median test, p = 0.001). Delayed Diagnosis was found in 28/47 (62%) of children and was observed more in unilateral than bilateral children (67% vs 52% respectively). The most common causes of delayed diagnosis are misdiagnosis by the first examining ophthalmologist (25%) followed by parental misjudgment of the child’s complaints (21%).
Referral delay
The median referral time was 3 weeks (0–7 weeks) for unilateral patients and 4 weeks (0–8 weeks) for bilateral patients. The referral time was longer in bilateral children (Mood’s median test, p = 0.07). Delayed referral was found in 27/47 (60%) of children and was observed more in bilateral than in unilateral children (61% vs 54% respectively). The most common causes of delayed referral were shopping for a second opinion after the initial proper diagnosis (20%) followed by improper explanation of the child’s diagnosis to the parents (16%).
Total delay
The median delay time was 9 (range 3–16) weeks for unilateral patients and 5 (range 2–11) weeks for bilateral patients. The delay time was significantly longer in unilateral children (Mood’s median test, p = 0.003). Total delay was found in 26/47 (58%) children, more in unilateral than in bilateral children (67% vs 43% respectively). The causes of total delay >2 months are summarized in Table 4. The causes were divided into two main categories: (a) delayed diagnosis and (b) delayed referral.
Table 4.
Diagnosis and referral delay times and causes in the studied cohort.
| Unilateral |
Bilateral |
Total |
|||||
|---|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | ||
| Diagnosis delay | |||||||
| ≤2 weeks | 8 | 33 | 11 | 48 | 19 | 42 | |
| >2 weeks | 16 | 67 | 12 | 52 | 28 | 62 | |
| Referral delay | |||||||
| ≤2 weeks | 11 | 46 | 9 | 39 | 20 | 44 | |
| >2 weeks | 13 | 54 | 14 | 61 | 27 | 60 | |
| Total delay | |||||||
| ≤2 months | 8 | 33 | 13 | 57 | 21 | 47 | |
| >2 months | 16 | 67 | 10 | 43 | 26 | 58 | |
| Total delay >2 months and advanced disease | |||||||
| Number | 16 | 100 | 10 | 100 | 26 | 100 | |
| p-value | 0.006* | 0.007* | 0.00002* | ||||
| Causes of delayed diagnosis | |||||||
| Misdiagnosis | 4 | 25 | 3 | 25 | 7 | 25 | |
| Parental misjudgment | 3 | 19 | 3 | 25 | 6 | 21 | |
| Social factors | 3 | 19 | 2 | 17 | 5 | 18 | |
| Economic factors | 3 | 19 | 3 | 25 | 6 | 21 | |
| Unknown | 3 | 19 | 1 | 8 | 4 | 14 | |
| Total | 16 | 100 | 12 | 100 | 28 | 100 | |
| Causes of delayed referral | |||||||
| Second opinion shopping | 4 | 31 | 5 | 36 | 9 | 20 | |
| Misexplanation to parents | 3 | 23 | 4 | 29 | 7 | 16 | |
| Paternal misjudgment | 2 | 15 | 1 | 7 | 3 | 7 | |
| Social factors | 1 | 8 | 1 | 7 | 2 | 4 | |
| Economic factors | 3 | 23 | 2 | 14 | 5 | 11 | |
| Unknown | 0 | 0 | 1 | 7 | 1 | 2 | |
| Total | 13 | 100 | 14 | 100 | 27 | 60 | |
Statistically significant.
The children with Total delay >2 months was significantly associated with advanced disease in at least one eye (p = 0.006, 0.007, 0.00002, unilateral, bilateral and whole group respectively) (Table 4).
Misdiagnosis
Two children were referred to AOO clinic by the misdiagnosis of Coats’ disease and the remaining children were referred to as retinoblastoma. Additional five children were initially misdiagnosed (2 cataracts, 1 Coats’s disease, 1 persistent fetal vasculature and one as bilateral chorioretinal coloboma) before getting a second opinion where a proper diagnosis was made leading to referral to the AOO clinic.
Discussion
In Alexandria, improved diagnosis and treatment of retinoblastoma started with implementing guidelines and Telemedicine with Jules-Gonin Hospital in Lausanne University, Switzerland. In 2007, a pilot prospective study for treatment on 16 children showed that 56% of children were unilateral and 44% were bilateral at presentation with the mean age at presentation 17 and 13 months respectively. Advanced disease (Reese-Ellsworth groups IV and V) presented in 54% of eyes.9 There was limited clinical information regarding presenting manifestation as the study focused mainly on treatment outcomes. Another prospective cohort in Alexandria (2008–2011)10 evaluated treatment protocols with respect to presenting IIRC6 group and laterality. It included 52 children, 63% unilateral and 11% extra-ocular disease. The mean age at presentation was 22 and 11 months for unilateral and bilateral children respectively. Advanced intraocular disease was present in 82%, 68% and 72% in unilateral, bilateral and all children, respectively, with leukocorea as the main presenting symptom. The current study shows observed less advanced intraocular disease from 72% to 64% of eyes and extra-ocular disease from 6% to 0%. Despite lower rate of advanced intraocular disease and absent extra-ocular disease, delayed diagnosis is still evident.
A larger prospective study (2007–2012)12 from the national cancer institute in Cairo included 262 cases with 57% unilateral children. The mean age on presentation was 26 and 19 months for unilateral and bilateral children respectively. Leukocoria presented the main complaint in 74% of children and 63% of eyes were classified as advanced intraocular disease with no child with extra-ocular disease. These results are similar to the current results from Alexandria making generalization of these results on whole Egypt justified as these are the two major cities in Egypt.
Retinoblastoma presentation varies, in Developed countries, retinoblastoma usually presents with leukocoria and/or strabismus while the tumor is still intraocular while in Developing countries, 60–90% of children present with extra-ocular tumors, proptosis and metastatic disease.13, 14
The successful management of retinoblastoma depends on the ability to detect the disease while it is still intra-ocular.1 Disease stage correlates with diagnostic delay, growth and invasion sequentially, followed by extra-ocular extension when the tumor reaches large intraocular size. Despite being intraocular most often in our data and the published literature from Egypt,12 advanced intraocular presentation is the main presentation reflecting a diagnostic delay.
This study is limited by the small number of cases, being retrospective, non-comparative and single institution review. A major limitation is the absence of correlation of advanced disease to success outcomes which are relative according to how the retinoblastoma multidisciplinary team prioritizes them. If life salvage and prevention of metastasis are prioritized as the success outcome enucleation would be the treatment option and advanced intraocular disease might have minimal impact on this success outcome. On the other hand, if eye and vision salvage are prioritized as a success outcome, more aggressive prolonged treatment options will be necessary and advanced presenting intraocular disease would have a demonstrable impact on eye salvage. Life salvage is our main aim in our institution. So, enucleation still plays a larger role in the treatment of retinoblastoma. We might consider this as a success to treat the cancer and prevent metastasis but might also be considered by others as a failure due to loss of eye and vision. In the current cohort, only one child developed metastasis with no deaths and correlating this to advanced presentation would be misleading.
In our study, Diagnostic delay was more significant in unilateral children, while referral delay was more significant in bilateral children. As a tertiary referral center, it was preferred to take the date of initial referral as an objective accurate time point rather than taking the initial parental complaint that is a subjective point. In our culture, there is tendency toward either exaggeration or minimization of duration of complaints either to gain more attention or to avoid blame. This reduces reliability on duration given by parents.11 Despite this we tried to outline both delay points. Physician related factors of delay were mainly related to misdiagnosis (25%) causing diagnostic delay and improper explanation of the diagnosis (16%) causing referral delay. Parental factors of delay were more pronounced in both diagnostic and referral delay mainly parental misjudgment of child’s complaints and shopping for a second medical opinion due to lack of trust in the primary ophthalmologist or for search for a better diagnosis. This shows that poor general education for parents and limited medical education about retinoblastoma for primary healthcare providers are the main contributors to delayed diagnosis and referral.
The first contact physician ability to identify the problem with proper explanation to the family and to make the appropriate timely referrals is crucial. Lack of proper medical diagnostic knowledge would be a significant barrier resulting in the higher possibility of metastatic disease and mortality. This emphasizes the importance of educational initiatives targeting primary healthcare providers.14 Retinoblastoma educational and public awareness campaigns increase case referrals and decrease advanced disease presentation, thus improving final outcomes.15, 16
Late diagnosis may be related to socioeconomic factors mainly poverty, limited healthcare access, distant residence, family problems and low socioeconomic status, as socioeconomic factors were found to affect treatment decisions either enucleation or eye salvage treatment,11 The current study shows that socioeconomic factors played a role in delaying both diagnosis and referral. Sometimes, parents can't afford transportation costs due to far distance between patients and treatment centers.17 Cost‐effectiveness of retinoblastoma treatment is still under studied,18 but measures targeting early diagnosis will be the way to reduce costs.
Screening for leukocoria was proposed for earlier detection but high false positive made it ineffective; this is more pronounced in developing countries with shortage of properly trained specialists in ophthalmology or pediatrics.19, 20 Parents or relatives are generally the first individuals to detect leukocoria in a child and their observation often initiates diagnosis.21 Flash photography is a useful tool in early identification of leukocoria and health awareness regarding its importance may be beneficial.
In developing countries, proper genetic counseling for parents and retinoblastoma survivors is an important strategy to improve early diagnosis; stressing on the genetic nature of retinoblastoma and the importance of screening the offspring of individuals affected by retinoblastoma by regular ocular examinations under anesthesia, beginning shortly after birth and continuing up to the age of 7 years.22, 23
Other proposed strategies to overcome delay presentation may be using social media for public awareness of retinoblastoma symptoms, screening of children at time of routine vaccination especially for red reflex, improvement of health insurance resources to cover treatment costs, increased training courses of ophthalmologists, improved research and introduction of instruments for diagnosis and treatment.1, 11, 23, 24
It is recommended that healthcare planning should include measures to improve ophthalmologic screening for at-risk neonates, ensure proper management of emotional reactions to both diagnosis and treatment to prevent shopping for second opinions and provide health education and promotion opportunities for surviving retinoblastoma patients concerning education, psychological support, occupational training, cosmetic rehabilitation and genetic counseling.25 Setting national strategic guidelines for nationwide retinoblastoma management, encompassing the aforementioned factors, was successfully done in Canada22 and Kenya26.
In Conclusion, delayed diagnosis in retinoblastoma results in more advanced intraocular disease which might complicate treatment, with poor eye and vision salvage and/or increase the rate of enucleation to prevent tumor spread. This is a problem that needs highlighting for its etiology and possible interventions. Collaborative multidisciplinary work on the national level is recommended to ensure better care.
Financial disclosure
None.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgment
The authors would like to acknowledge Dr. Brenda Gallie for her help in reviewing this manuscript and improving its English language presentation.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Dimaras H, Corson TW, Cobrinik D, et al. Retinoblastoma. Nature Reviews Disease Primers. 2015: 15021. [DOI] [PMC free article] [PubMed]
- 2.Othman I.S. Retinoblastoma major review with updates on Middle East management protocols. Saudi J Ophthal: Official J Saudi Ophthalmol Soc. 2012;26(2):163–175. doi: 10.1016/j.sjopt.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kivela T. The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. Br J Ophthalmol. 2009;93(9):1129–1131. doi: 10.1136/bjo.2008.150292. [DOI] [PubMed] [Google Scholar]
- 4.Dimaras H., Kimani K., Dimba E.A. Retinoblastoma. Lancet. 2012;379(9824):1436–1446. doi: 10.1016/S0140-6736(11)61137-9. [DOI] [PubMed] [Google Scholar]
- 5.Shields C.L., Shields J.A. Basic understanding of current classification and management of retinoblastoma. Curr Opin Ophthalmol. 2006;17(3):228–234. doi: 10.1097/01.icu.0000193079.55240.18. [DOI] [PubMed] [Google Scholar]
- 6.Murphree A.L. Intraocular retinoblastoma: the case for a new group classification. Ophthal Clin North Am. 2005;18:41–53. doi: 10.1016/j.ohc.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Soliman S.E., Dimaras H., Khetan V. Prenatal versus Postnatal Screening for Familial Retinoblastoma. Ophthalmology. 2016;123(12):2610–2617. doi: 10.1016/j.ophtha.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 8.Mallipatna A, Gallie BL, Chévez-Barrios P, et al. Retinoblastoma. In: Amin MB, Edge SB, Greene FL, editors. AJCC Cancer Staging Manual. Vol 8th Edition. New York, NY: Springer; 2017: 819–31.
- 9.Bessa A.S.S.A., Shakankiry N., Soliman S.E. Eye Preservation Approach in the management of retinoblastoma: a prospective study. Delta J Ophthalmol. 2007;8:8. [Google Scholar]
- 10.Soliman SE, Gomaa S, Galal MA, Souka AA. Evaluation of a proposed management protocol for retinoblastoma cases. 2011: 215.
- 11.Soliman S.E., Dimaras H., Souka A.A., Ashry M.H., Gallie B.L. Socioeconomic and psychological impact of treatment for unilateral intraocular retinoblastoma. J Francais D Ophtalmol. 2015;38:550–558. doi: 10.1016/j.jfo.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 12.El Zomor H., Nour R., Alieldin A. Clinical presentation of intraocular retinoblastoma; 5-year hospital-based registry in Egypt. J Egypt National Cancer Inst. 2015;27(4):195–203. doi: 10.1016/j.jnci.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Chantada G.L., Qaddoumi I., Canturk S. Strategies to manage retinoblastoma in developing countries. Pediatric Blood Cancer. 2011;56(3):341–348. doi: 10.1002/pbc.22843. [DOI] [PubMed] [Google Scholar]
- 14.Leal-Leal C.A., Dilliz-Nava H., Flores-Rojo M., Robles-Castro J. First contact physicians and retinoblastoma in Mexico. Pediatr Blood Cancer. 2011;57(7):1109–1112. doi: 10.1002/pbc.23227. [DOI] [PubMed] [Google Scholar]
- 15.Leander C., Fu L.C., Pena A. Impact of an education program on late diagnosis of retinoblastoma in Honduras. Pediatr Blood Cancer. 2007;49(6):817–819. doi: 10.1002/pbc.21052. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Galindo C., Wilson M.W., Chantada G. Retinoblastoma: one world, one vision. Pediatrics. 2008;122(3):e763–770. doi: 10.1542/peds.2008-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Hadad S.A., Al-Jadiry M.F., Al-Darraji A.F., Al-Saeed R.M., Al-Badr S.F., Ghali H.H. Reality of pediatric cancer in Iraq. J Pediatr Hematol Oncol. 2011;33(Suppl 2):S154–156. doi: 10.1097/MPH.0b013e318230e218. [DOI] [PubMed] [Google Scholar]
- 18.Aziz H.A., Lasenna C.E., Vigoda M. Retinoblastoma treatment burden and economic cost: impact of age at diagnosis and selection of primary therapy. Clin Ophthalmol. 2012;6:1601–1606. doi: 10.2147/OPTH.S33094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcou V., Vacherot B., El-Ayoubi M., Lescure S., Moriette G. Abnormal ocular findings in the nursery and in the first few weeks of life: a mandatory, yet difficult and neglected screening. Arch Pediatr. 2009;16(Suppl 1):S38–41. doi: 10.1016/S0929-693X(09)75299-6. [DOI] [PubMed] [Google Scholar]
- 20.Wagner R.S. Difficulties in screening for retinoblastoma. J Pediatr Ophthalmol Strabismus. 2005;42(4):204. doi: 10.3928/01913913-20050701-16. [DOI] [PubMed] [Google Scholar]
- 21.Abramson D.H., Beaverson K., Sangani P. Screening for retinoblastoma: presenting signs as prognosticators of patient and ocular survival. Pediatrics. 2003;112(6 Pt 1):1248–1255. doi: 10.1542/peds.112.6.1248. [DOI] [PubMed] [Google Scholar]
- 22.National Retinoblastoma Strategy Canadian Guidelines for Care / Stratégie thérapeutique du rétinoblastome guide clinique canadien. Can J Ophthalmol. 2009; 44(Supp 2): S1-88. [DOI] [PubMed]
- 23.Soliman S.E., ElManhaly M., Dimaras H. Knowledge of genetics in familial retinoblastoma. Ophthal Genet. 2016;1–7 doi: 10.1080/13816810.2016.1195846. [DOI] [PubMed] [Google Scholar]
- 24.Alfaar A.S., Nour R., Bakry M.S. A change roadmap towards research paradigm in low-resource countries: retinoblastoma model in Egypt. Int Ophthalmol. 2016 doi: 10.1007/s10792-016-0233-4. [DOI] [PubMed] [Google Scholar]
- 25.Patenaude A.F., Kupst M.J. Psychosocial functioning in pediatric cancer. J Pediatric Psychol. 2005;30(1):9–27. doi: 10.1093/jpepsy/jsi012. [DOI] [PubMed] [Google Scholar]
- 26.Kenyan Ministry of Health. Kenya national retinoblastoma strategy: Best practice guidelines; 2014. <http://www.health.go.ke/images/downloads/Best-Practice-September-2014.pdf>.


