Abstract
Background:
Global aquaculture production has increased continuously over the last five decades, and particularly in China. Its aquaculture has become the fastest growing and most efficient agri-sector, with production accounting for more than 70% of the world’s aquaculture output. In the new century, with serious challenges regarding population, resources and the environment, China has been working to develop high-quality, effective, healthy, and sustainable blue agriculture through the application of modern biotechnology. Sound knowledge related to the biology and ecology of aquatic organisms has laid a solid foundation and provided the innovation and technology for rapid development of the aquaculture industry. Marine biotechnology, which is enabling solutions for ocean productivity and sustainability, has been promoted since the last decades of the 20th Century in China.
Objective:
In this article, priority areas of research, mainly genetic breeding, omics studies, novel production systems, biosecurity, bioprocesses and biorefinery, as well as the major progress of marine biotechnology R&D in China are reviewed.
Conclusion:
Current innovative achievements in China are not enough and the level and frequency of academic advancements must be improved. International cooperation and assistance remain crucial for the success of marine biotechnology.
Keywords: Aquaculture, Marine biotechnology, Genetic breeding, Omics study, Novel production systems, Biosecurity, Bioprocesses and biorefinery, China
1. Introduction
China has more than 18,000 km of coastline, over 6,500 islands, and the jurisdiction of about 3 million km2 of sea area, in accordance with the “exclusive economic zone” and “continental shelf” systems defined in the “United Nations Convention on the Law of the Sea”. These vast coastal areas and territorial seas have world-renowned fishing grounds, rich in economically valuable marine resources. Food safety is one of the most important issues to the Chinese people, whose current population is more than 1.3 billion and predicted to reach 1.6 billion by 2030. The terrestrial resources of China are limited. In particular the per capita land-based natural resources suitable for agriculture (0.1 ha/per capita) are much lower than the world's average (0.3 ha/per capita) [1]. To meet the ever-growing food requirement for the continuously increasing population, food security is one of the most important issues facing the Chinese people [2].
Global aquaculture production has increased continuously over the last five decades and, particularly in China, aquaculture has become the fastest growing and most efficient agri-sector, with production accounting for more than 70% of the world’s aquaculture output [3]. Traditionally, China has been a global leader in fisheries production. Since the 1950’s fresh water aquaculture in China has been rapidly developed while mariculture only began to grow quickly after the 1980’s. The cultured seafood production in China reached 1,100,000 tons in 1987. Six main kinds of cultured seafood including kelp, shrimp, mussel, razor clam and clam ranked first globally in the same year [4]. China’s aquaculture production in 2006 reached to 14,456,400 t, the first time it surpassed marine fish capture production (14,420,400 t), with a more reasonable species proportion [5].
2. Aquaculture and Marine Biotechnology
2.1. Status of Aquaculture in China
In 2013, China’s aquatic products output was the largest in scale in the world. China’s aquaculture production reached 45.42 million t, accounting for 73.59% of total fisheries output (61.72million t) [6]; China’s fishing trade accounts for 6.8% of global exports, with the exports ranking first or second in the world, and imports being tenth [3]. There are 20.65 million Chinese people whose lives depend on fisheries and aquaculture, and 14.43 million of fishing labor force (fishermen), accounting for 2% of the agricultural population in China. Aquatic production provided the Chinese with almost 1/3 of their animal protein, which greatly reduced protein production pressures, prevented a future food crisis, and played an important role in stabilizing prices. It is expected that the total output of aquatic products in 2030 will reach 76 million t. At present, China has become the only country where the output of the mariculture industry is larger than that of the fishing industry. Marine fisheries GDP was 1024.9 billion Yuan in 2014, equivalent to 1.61% of the gross domestic product over the same period [7].
Having developed for 20 years, China’s marine fishery progressed from solely capture and aquaculture production, to gradually extending to including aquaculture, aquatic products in-depth processing, pharmaceuticals, and other high-value-added industries. The extension of the marine industrial chain clearly indicated the expansion of production and increase of benefits, promoting greatly the growth of marine economy [8]. China is now actively promoting the integration and innovation of fisheries sciences, leading fisheries science to a new stage of development [9]. However, a wide gap still exists between China and the world’s advanced countries and regions at the level of fishery science and technology. The following three figures provide some statistics for national marine fisheries (including mariculture) [6].
2.2. Opportunities and Challenges of Aquaculture Development in China
Aquaculture is the fastest growing food-producing sector in the world and is expected to continue to grow and help compensate for the anticipated global supply shortage of capture fisheries [10]. To feed the estimated world population of 9 billion people predicted in 2050, food output must increase by 70%. Obviously, the only way to meet the increasing demand for fish is through aquaculture. Daniel Cressey [11] explored the challenges for fish farmers and what it means for dinner plates in 2030. However, sustainable development of the aquaculture industry faces difficult issues, including lack of captive breeding and domesticated strains, disease control, remediation of polluted water environments, the changing globe climate, and so on. Therefore, development of knowledge-based technologies is urgently needed.
China’s rapid socio-economic development has also exerted increasing pressure on the coastal ecosystems with a series of unusual ecological phenomena, such as harmful algal blooms, jellyfish outbreaks, Enteromorpha blooms [7], declines in fishery resources and so on, which indicates that the Chinese coastal ecosystems are in a period of significant change. Because of the scale of the mariculture industry in China, the types of mariculture practiced and the cultured organisms produced, the impacts of mariculture on the marine environment and the Chinese ecosystems’ responses are unique in the international arena. For these reasons, mariculture in China has been of concern to the international scientific and industrial communities. It is viewed as highly likely to significantly affect the services and the values of the coastal ecosystem [2].
Another environmental challenge is the interference by human activities. Currently, the coastal zone is the region most affected by human activities, with pollution, eutrophication, changes in sediment transport, urbanization, land reclamation, over-fishing, tourism, and mining; these activities constantly threaten the health of coastal ecosystems. China is located on the western side of the Pacific Ocean, with coastal areas on its eastern and southern sides and more than 18,000 km of shoreline. The total area of coastal zone is about 285,000 km2, including over 6000 islands that each area is larger than 500 m2. Chinese maritime regions are in the middle and low latitudes with distinctive natural environments and resources. However, while rapid economic growth in coastal zones created enormous economic benefits, it also resulted in environment damage to natural areas and adversely influenced the sustainable development of coastal mangroves, corals/coral reefs, spawning grounds in estuaries and gulfs, and different sub-ecosystems [2].
At present and for a fairly long time to come, global-warming leading to environmental change will not only be a hot scientific issue, but also a major political issue of international concern. Thus far, the environmental quality of China’s coastal waters is good overall; however, the inshore zones have been polluted to different degrees in some estuaries, bays, ports, and adjacent sea areas of the large and medium-sized cities and major industrial areas. In 2014 [7], the overall level of pollution in Chinese coastal waters was still relatively high. Roughly 150,780 km2 of the nation’s seawater does not meet the clean water quality standards. Pollutants from most of the sewage-discharging sites exceeded the standard for waste water discharge. In addition, the rapid but sometimes unregulated development of the mariculture industry causes negative impacts on coastal ecosystems, leading to problems such as pollution, eutrophication, change of ecosystem structure and function, disease, red tide, etc. Within the marine ecosystems monitored by SOA, including estuaries, coastal bays, beaches, reefs, mangroves and sea grass beds, sub-healthy and non-healthy sub-ecosystems accounted for 71% and 10% respectively [7]. Solving these problems is an important task for scientists in China now and in the future.
2.3. Marine Biotechnology Enabling Solutions for Ocean Productivity and Sustainability
Sound knowledge of the biology and ecology of aquatic organisms has laid a solid foundation and provided the innovation and technology for rapid development of the aquaculture industry and other novel products. The application of biotechnology to freshwater and marine resources may help to address the global challenges of food, energy security and health as well as contribute to green growth and sustainable industries [12].
In the new century, with serious challenges regarding population, resources and the environment, China has been working to develop high-quality, effective, healthy, and sustainable blue agriculture through the application of modern biotechnology [13].
Marine biotechnology has been developed since the last decades of the 20th Century in China. At the end of the 1980’s, some foresighted Chinese scientists, led by Prof. C.K. Zeng, began to suggest that the government pay close attention to marine biotechnology [4]. Through several years of effort by the scientists and administrators, the subject of Marine Biotechnology gained approval for listing in the National High Tech Project (863 Project) in 1996. The subject’s mission focuses on the strategic, foresighted and most advanced marine biotechnology that benefits China's long and medium-term development needs, and thereby promotes the development of innovative technologies and fosters growth of the high-tech marine industry. The objectives are thus to promote the development of key technologies for rational utilization of marine bio-resources, as well as environmental protection technologies that relate to social sustainable development [14].
During the first five years (from 1996 to 2000) of the 863 Project’s Marine Biotechnology listing about 97.5 million RMB were funded by the Chinese government and additional funds of 87.5 million RMB were matched by local governments and enterprises. 81 institutions, the majority of which were located in Shandong, Beijing and Guangdong, were involved in the subject. Actually, the marine biotechnology project impelled a lot of national R&D institutions to initiate research in this field. Moreover, biotech scientists who had been conducting terrestrial research were also attracted to the marine field. Thus, the distance between marine biotechnology and land-based biotechnology was greatly reduced. Afterward, during the successive three “Five-Plans” period (from 2001 to 2015), the Chinese government invested more and more R&D funds in marine technology. Through decades of effort using marine biotechnology, China has already attained many remarkable achievements.
3. Priority Areas and Major R&D Progress of Marine Biotechnology in China
3.1. Genetic Breeding
The precondition of aquaculture production is to continuously obtain different suitable seedlings, larvae or fingerlings. In ancient times, farmers collected wild larval or juvenile aquatic animals to culture. At present, there are still some farms that have to use wild seeds, for example eels and some high-price rock fish etc. However, more and more species have been domesticated and bred under artificially controlled conditions.
Manipulation of reproduction enables growers to intervene and control the reproductive procedure of animals by physical, chemical and biochemical means, in order to fit the needs of aquaculture [15, 16]. Genetic manipulation allows modification and recombination of the genetic structure at the cytological and molecular levels. Reproductive manipulation includes the regulation of maturation, control of gamete spawning, implementation of artificial fertilization and sex ratio control, while genetic manipulation includes hybridization, polyploid induction, gynogenesis and androgenesis, cell fusion, nuclear transplant and foreign gene transfer.
One of the most important factors for sustainable aquaculture is the development of high performance culture strains. Over the last 20 years, significant progress has been made in fish biology and biotechnology, especially in the field of genetic breeding [17]. The development and application of marine biotechnology may improve the genetic performances of aquatic animals with higher output, better quality and more resistance against disease.
Genetic breeding is a process that recodifies some heritable traits targeted to obtain neotype and/or improved varieties. For the purpose of genetic improvement, researchers can select for desirable genetic traits, integrate a suite of traits from different donors, or alter the innate genetic traits of a species [18]. These improved varieties have, in many cases, facilitated the development of the aquaculture industry by lowering costs and increasing both quality and yield [19].
There are many approaches to achieve genetic improvements for aquatic organisms, such as traditional selective breeding and molecular marker-assisted selective breeding [20], hybridization (close- and distant-), cytoengineering (including polyploidization, gynogenesis and androgenesis), genome-wide selective breeding, nuclear transplantation, transplantation of germline stem cells and germ cells [21], modification breeding technologies (transgenic technology) and so on.
Chromosome set manipulation is a useful genetic tool that can artificially change the composition of the normal chromosomal structure in the diploid animals, in order to obtain production gains such as higher growth rate, better resistance against diseases. Sex-control can be achieved via chromosome manipulation. Both physical (including temperature, pressure, etc) and chemical methods have been used to produce triploid and tetraploid stocks. The timing, magnitude and rate of application of temperature shock are the critical variables in successful triploid or tetraploid shrimp induction [22]. Triploids typically exhibit rapid growth, strong disease resistance and high yield, so they are of high economic value. Additionally, the majority of allotriploid fish are infertile, which reduces issues associated with diversion of energy to gametic growth, mixing of genetic lines, and interbreeding with wild stocks [18, 23].
Breeding of important culture animals has made important progress in China. From 1996 to 2011, 109 novel mariculture varieties have been approved by the National Certification Committee for Aquaculture Protospecies and Varieties (NCCAPV) [24]. These varieties were obtained by artificial selection, hybridization, introduction from foreign countries and so on, including fishes, molluscs (scallop, abalone, pearl oyster), crustaceans (shrimp and crabs), echinoderms (sea cucumbers, urchins) and sea weeds (kelp, nori and Gracilaria). Fig. (4) shows the rates of different approaches by which the 109 varieties were obtained [24].
Fig. (4).
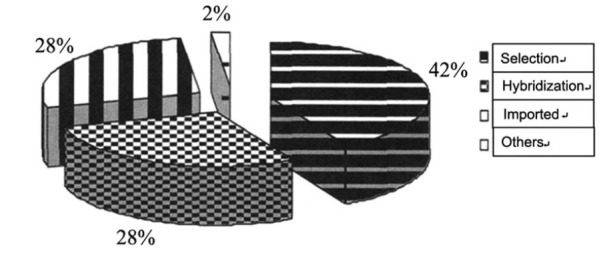
Rates of the methods used for novel aquatic varieties approved in China.
Since 2001, the National High-Tech Project of China have founded a key Program” Seed Engineering of Mariculture” through which many novel varieties have been selected. For example, in 2012, 2013 and 2014, nine, seven and fifteen novel aquatic varieties were approved in each year respectively. Table 2 lists major novel varieties approved by the NCCAPV with their primary merits. All of these novel varieties have been playing important roles to increase aquaculture production in China.
Table 2.
Recent progress of whole-genome sequencing.
| Species | Year |
1st &
Commu. Authors |
Genome Size
(C50: Contig N50, S50: Scaffold N50) |
References |
|---|---|---|---|---|
| Oyster (Crassostrea gigas) | 2012 | G.Zhang J.Wang |
559mb (C50: 19.4kb, S50:401kb) |
Nature Vol.490, 49-54. |
| Common carp, (Cyprinus carpio) | 2014 | P.Xu, X. Sun |
1.69 Gb (C50: 68.4kb, S50: 1mb) |
Nature Genetics Vol. 46 No 11: 1212-1221. |
| Tongue sole (Cynoglossus semilaevis) | 2014 | S.Chen J.Wang |
477Mb (C50: 26.5kb, S50:867kb) |
Nature Genetics Vol.46 No 3 253-260. |
| Large yellow croaker (Larimichthys crocea) | 2014 | C. Wu Y. Liu |
728Mb (C50:25.7kb, S50: 498.7kb) |
Nature Communications DOI: 10.1038/ncomms6227, 1-7. |
| Large yellow croaker (Larimichthys crocea) | 2015 | J. Ao X.Chen |
679Mb (C50:63.1kb, S50:1.03Mb | PLOS Genetics 11(4): e1005118. DOI:10.1371/journal.pgen.1005118 |
| Saccharina japonica (Kelp) | 2015 | H.Ye F.Zhao |
537Mb (C50:58.87kb S50: 252.0kb) |
Nature Communications |6:6986 | DOI:10.1038/ncomms7986 |
| Ctenopharyngodon idellus (Grass carp) | 2015 | Y.Wang Z.Zhu |
0.9Gb(F) (C50:40.78kb, S50: 6.46 Mb) 1.07 Gb(M) (C50:18.3kb, S50: 2.28 Mb) |
Nature Genetics (advance online) Doi:10.1038/ng.3280 |
For example, Wang et al. isolated two pairs of Y and X chromosome-specific markers from yellow catfish (Pelteobagrus fulvidraco) by AFLP and SCAR screening, which then were utilized for screening YY super-male individuals. Subsequently, they developed a Y- and X-specific allele marker-assisted sex control process for cultivating all-male populations [17]. This artificially selected stock with stable genetic traits has been approved as a novel variety, referred to as “yellow catfish all-male No. 1” by NCCAPV, because yellow catfish males grow faster than females, and generate individual differences in total size of about 2-3 fold. Because transgenic methods can be easily applied to incorporate the function of a specific gene, this approach is potentially the most direct and rapid method of obtaining a stable and genetically inherited trait in fish. The first transgenic fish was generated by over-expression of human growth hormone (hGH) gene driven by a mouse metallothionein-1 (MT) gene promoter in Chinese goldfish [25]. Since that time, a lot of progress has been made in China. In addition to transgenesis, recently developed genome editing techniques provide an enormously valuable tool for fish breeding that allows researchers to modify multiple genes at precise sites with high efficiency and in a comparably short time [26].
3.2. Omics Study
Fish biology and biotechnology have provided the innovation and technology for rapid development of the aquaculture industry. With regard to marine biology, China has conducted a great deal of research in basic theory, resources investigation, important species cultivation, domestication of varieties, artificial breeding, and culture technology. China has also gradually built the world’s largest algae, shrimp, shellfish and fish aquaculture industries.
It is generally agreed that the application of ‘omics’ technologies in aquatic organisms, as in other farming activities, may significantly contribute to advancement of the aquaculture industry. In recent years, various experimental ‘omics’ approaches have been applied to farmed organisms to increase the genomic resources available. These tools are utilized to identify genetic markers associated with traits of commercial interest, and to unravel the molecular basis of different physiological processes. In recent years, an important effort has been directed towards the use of functional genomics, proteomics and metabolomics to better characterize the reproduction, development, nutrition, immunity and toxicology of aquaculture organisms.
Special attention is devoted to genomics, transcriptomics, proteomics, metabolomics and nutrigenomics [27-30]. It is hoped that new bio-information will contribute to increased success in the marine sciences and related industries [31].
Recent advances in genomic and post-genomic technologies have now established the new standard in medical and biotechnological research. The introduction of next-generation sequencing (NGS) has resulted in the generation of thousands of genomes from all domains of life [32]. Genomic and metagenomic analyses of marine organisms and the marine environment are revealing the complexity and biodiversity of marine ecosystems [33].
China’s marine functional genomics and genetics research had already started at the beginning of the 21st Century and thus far has catalyzed important progress in functional gene isolation, cloning and application to marine bio-economic organisms. China took the lead within the international community in obtaining expression of a large number of expressed sequence tags (EST) for shrimp, scallops and other marine organisms; completed, for the first time in the world, the whole-genome sequencing of White Spot Syndrome Virus (WSSV) in shrimp [34]; determined several fish Iridovirus genome sequences [35-39]; established specific genetic databases of China’s marine bioresources by bioinformatics techniques, laying a foundation of property rights and technology for securing marine functional genomics resources and promoting industrialization.
Whole-genome sequencing is one of the most challenging applications of NGS to accelerate biological and applied research in non-model aquatic species. Some noteworthy progress has been made in recent years in China (Table 2). In 2012, the oyster genome sequencing and assembly were completed, which revealed stress adaptation and complexity of shell formation [40]. In the same year, Xu et al. [41] reported genome sequence and genetic diversity of the common carp, Cyprinus carpio. Chen et al. [42] reported the whole genome sequences of the flatfish Cynoglossus semilaevis, which provided insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Later, Wu et al. [43] published a draft genome of a large yellow croaker Larimichthys crocea, which revealed well-developed innate immunity, while Ao J et al. [44] reported a better assembled genome sequencing of the same species that provided insight into the molecular and genetic mechanisms of stress adaptation. Recently, genome sequencing of a kelp Saccharina japonica and the grass crap Ctenopharyngodon idellus have been published by Ye H et al. [45] and Wang Y et al. [46], respectively. These results provide novel insight into kelp biology and may help us understand the evolution and vegetarian diet adaptation of the grass carp. The availability of these genomic data have opened up a number of opportunities to advance basic research and develop commercial applications.
Genetic linkage maps are powerful tools in breeding programs and genome evolution studies. Knowledge of the genetics and physiology of economically important marine organisms remains scarce. For some purposes, such as genetic breeding programs, the application of genomics may be a faster approach than the de novo sequencing of genomes and further identification of genes and genetic markers. The construction of genetic linkage maps may allow a relatively rapid recognition of quantitative trait loci (QTLs) for marker-assisted selection (MAS) in aquatic organisms [20]. Genetic linkage maps, the necessary framework for any MAS program, have been constructed for over 40 aquaculture species and are currently being constructed for additional species [47]. In China, the genetic linkage maps for sea weeds, shrimp, crabs, molluscs, echinoderms and fishes, such as Litopenaus vananmei [48], Fenneropenaeus chinensis [49], Portunus trituberculatus [50], Chlamys farreri [51, 52], Crassostrea virginica [53], Haliotis discus hannai [54], Apostichopus japonicas [55], Strongylocetrotus nudus [56], Larimichthys crocea [57], Epinephelus bruneus [58] etc., have been successively constructed.
Growth traits including growth rate, body weight and length are the most important traits in aquaculture species [19]. They can be easily measured. Due to their high heritability in most culture species, they can be improved using traditional selection methods. QTL analysis for growth traits has been conducted in many species. For example, Song W. et al. [59] constructed high-density genetic linkage maps and mapped growth-related QTLs on it for the Japanese flounder (Paralichthys olivaceus). Song W. et al. [60] constructed a high-density microsatellite genetic linkage map and mapped sexual and growth-related traits on it in half-smooth tongue sole (Cynoglossus semilaevis). Liu L. et al. [50] identified QTLs for growth-related traits in the swimming crab Portunus trituberculatus.
In terms of value, shrimp are the most important seafood product traded globally, with production from aquaculture estimated at 3.1 million metric tonnes and valued at US$12 billion in 2008 (Shrimp News International, 2009). Considerable effort has been expended on shrimp genome research since the end of the twentieth century. However, due to the great complexity of conducting a genome survey of Litopenaeus vannamei, its genome investigation is behind the progress of genome research for aquatic fishes, molluscs and sea weeds. Zhang X. et al. [61] reported construction and characterization of a bacterial artificial chromosome (BAC) library of the Pacific white shrimp, L. vannamei. A high density genetic map was constructed using a specific-locus amplified fragment sequencing (SLAF-seq) approach. In the constructed linkage map, a total of 6,146 markers spanning 4,271.43 cM were mapped to 44 sex-averaged linkage groups, with an average marker distance of 0.7 cM. The integration analysis linked 4,908 scaffolds from genome survey analyses and 1,504 BAC clones to the constructed linkage map. With the help of map integration, many markers were annotated. This high-density genetic linkage map reveals the basic genomic architecture and it will be useful for comparative genomics research, genome assembly and genetics-based breeding of L. vannamei and other penaeid shrimp [62].
3.3. Novel Production Systems
In the development of mariculture industries, paying attention solely to the economic benefits and ignoring the long-term ecological effects can be very detrimental because it can severely hinder sustainable mariculture development. For example, the ill-considered intensification of shrimp and scallop culture in years past caused the massive mortalities in China. So, present-day Chinese mariculture ought to adjust its industrial structure, to reduce environmental pollution and to maintain sustainable development.
There are multiple major aquatic production systems on basis of the eco-habitats, which include the following:
Pond production systems in land-based freshwater or on coastal beaches, for fish, shrimp, crab, sea cucumber and molluscs. Almost half of the total aquaculture yield comes from land-based ponds and water-based pens, cages, longlines and stakes in brackish water and marine habitats.
In-door production in freshwater or on coastal beaches for hatchery and grow-out of fishes, abalone, etc;
Suspended aquaculture in coastal water for sea weeds and scallops;
Cage culture in lakes or in the near-shore sea for fishes.
In view of the current situation, different kinds of cultural modes can clearly coexist. Extensive mariculture in tidal flat ponds and shallow seas are still playing main role in Chinese production. However, the conversion of coastal ecosystems to aquaculture ponds has significantly degraded the wetland and aquatic environments. In order to keep the balance of ecology and environment, i.e., to reduce the impact of cultural effluent on the seawater, integrated multi-trophic aquaculture (IMTA) in marine offshore systems has been studied and practiced, including the recycling of waste nutrients from higher trophic-level species into production of lower trophic-level crops of commercial value; co-location of cultural species, adjustment of the stocking density and utilization of formulated diets with more nutritious components and higher conversion efficiencies, etc [63-66]. In China, the two main forms of marine IMTA systems are sea-ranching and suspended aquaculture. For example, at Zhangzidao Island, which is comprised of nine islets in the northern Yellow Sea, there have been significant efforts to “optimize” or improve ecological conditions at the farm site, including propagation and planting of seaweeds and creation of artificial reefs. Many of the techniques employed at the site benefit from inter-specific relationships and existing infrastructure to co-culture a range of species. Suspended culture in Sungo Bay, in the eastern end of Shandong Peninsula, is one of the most important mariculture regions for scallop Chlamys farreri and kelp Laminaria japonica in northern China. The abalone H. discus hannai is also cultured here, and to a lesser degree blue mussel M. edulis. During the culture of Laminaria on long lines, abalones are grown in lantern nets hung vertically from the lines, thus allowing the abalone to feed directly on the kelps. Following harvest of Laminaria, abalones are fed with dried Laminaria until the next crop matures.
Since the late 1990’s, a new shrimp production system, the so-called “High-Located Pond” system, has been becoming more popular, especially in the southern coastal provinces of China (Fig. 5). This type of pond is typically constructed with a plastic sheet liner on its bottom to facilitate the removal of the polluted sediments. Increasing stocking densities, in combination with more effective pumping and aeration of the water and better system management produces yields that are higher by a factor of 3-4.
Fig. (5).

A typical intensive shrimp culture system (High-Located Pond) in Hainan Province.
Most recently, indoor intensive culture using recirculating aquaculture systems (RAS) has been rapidly developing in China (Fig. 6). The RAS approach is expected to result in higher yields and lower or null effluents. Fig. (7) shows some new RAS facilities and equipment developed by IOCAS.
Fig. (6).
Modern Industrial Recirculating Aquaculture Systems (RAS).
Fig. (7).
Some new RAS facilities and equipment have been developed by IOCAS.
High-density rearing of aquatic animals typically requires some waste treatment infrastructure. At its core, biofloc is a waste treatment system [67]. Recently, the biofloc approach has been utilized in Chinese aquaculture production and has shown good results [68-70].
In addition, China is also intensively developing cage mariculture. At present, the most utilized shallow sea culture area in China is within 15m depth. Not surprisingly, this sea area is also the most impacted zone from land sources. In order to realize the sustainable development of shallow sea Chinese aquaculture in the new century and minimize the adverse impacts of mariculture on shallow sea area, the fish farming range should be increased to include more offshore areas. Fish farming sites ought expand to 20m depth and some sea areas could possibly support culture at 30-40m depth. The corresponding advanced cultivating and engineering facilities should be made for the future offshore mariculture. The main cultural species number about 40, including perch, turbot, flounder, etc. in the north of China and large yellow croaker, red drum and perch, etc. in the south. The total area of mariculture cages has reached 21,930,000 m2 and the total production in China was about 305,610t in 2008 [6]. Now modern net-cages and relative facilities suitable for deep-sea and typhoon-resistant aquaculture in China’s coastal waters have been developed. Large and deep-setting anti-wave cage technology has developed very quickly, with good applications for large yellow croaker (Larimichthys crocea), cobia (Rachycentron canadum) and pompano (Trachinotus ovatus) [71].
Fig. (8) shows the locations of five novel production systems spread across the coastline of China.
Fig. (8).
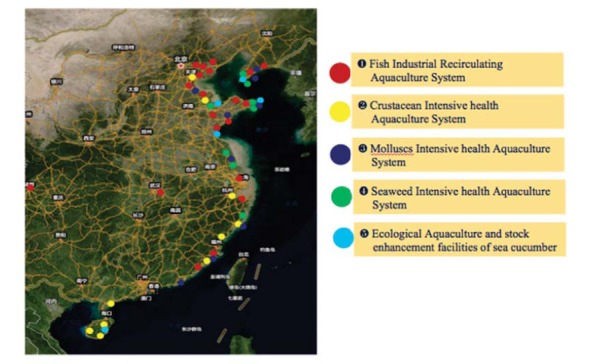
Five Novel Production Systems have been spread across the coastline of China.
3.4. Biosecurity
With the increase of living standards, economic globalization and the rapid worldwide growth in international trade, an increasing number of countries and international organizations have recognized the importance of biosecurity, which involves food safety, animal and plant health and well-being, and environmental risks. Therefore, the FAO of the United Nations has adopted biosecurity as one of the organization’s 16 priority areas of interdisciplinary action in medium-term programs, aiming to promote, develop and enhance a common strategy of food, agriculture, fisheries and forestry policy and management framework [72].
Biosecurity of marine aquaculture is concerned with the management of health and the environmental risks of marine aquaculture-related organisms. The risk includes all non-native species, the introduction of disease organisms and pathogenic microorganisms of cultured species, major infectious diseases destroying the biological diversity and cross-sectional diseases resulting in a decrease of large-scale biological production, as well as biological and environmental pollution problems caused by development of biotechnologies.
Knowledge of comparative immunology is crucial to our understanding of the defense mechanisms of different aquatic animals, such as fishes, shrimp, molluscs and others, against relevant pathogens. Two Major State Basic Research Development Programs in China (973 programs) which integrated several research teams from the Institute of Oceanology, Chinese Academy of Sciences; the Third Institute of Oceanology, State Oceanic Administration; Zhejiang University; SUN YAT-SEN University; and Shandong University were undertaken from 2000 until 2010 to focus on aquatic animal immunology and disease control. These studies made great progress on the immunology of shrimp, molluscs and fishes. A special issue on “Comparative Immunology in China” was invited for publication in Developmental and Comparative Immunology [73], six papers of which reviewed the major studies and advances in knowledge and its application of innate and adaptive immunology from shrimp, molluscs and fishes. The review paper “Advances in research of fish immune-relevant genes: A comparative overview of innate and adaptive immunity in teleosts” [74] has been one of the most cited in the DCI journal for quite some time.
Due to the importance of shrimp aquaculture in China, researchers have paid considerable attention to the molecular mechanisms of shrimp disease occurrence and tried to develop an efficient control strategy for disease. The first line of defense against microbial infections in animals is the innate immune response, which triggers diverse humoral and cellular activities via signal transduction pathways [75]. Based on the recent data, a brief outline of the immune system of shrimp was drawn, which will help us to understand the immune responses of shrimp to different pathogens [76].
For this industry, viral infection poses a significant threat and causes heavy economic losses. Simple, rapid and accurate methods for detecting pathogens directly from aquatic animal samples are therefore crucial for monitoring the infected fishes and minimizing economic losses. Molecular technology of pathogen detection has been established in China for a series of fish, shrimp, and scallops [77-82].
The most realistic approach to combat diseases is combining good husbandry and good feed with the use of prophylactic agents, including immunostimulants and probiotics [83-85].
Edwardsiella tarda, Vibrio, Streptococcus iniae, and Pseudomonas fluorescens are severe bacterial pathogens in a wide range of farmed freshwater and marine fish. Against these pathogens, Sun’s team constructed multiple different types of monovalent and multivalent vaccines, including DNA vaccines, attenuated vaccines, subunit vaccines, and inactivated vaccines [86-90]. They have designed and developed DNA vaccines and subunit vaccines delivered by live attenuated pathogens via natural infection, which induces cross-genus protection. These vaccines induce effective protection against pathogens and have good application prospects.
Over the past few decades, Asian aquaculture production has intensified rapidly through the adoption of technological advances, and the use of a wide array of chemical and biological products to control sediment and water quality and to treat and prevent disease outbreaks [91].
Seafood safety is a crucial issue in the consumption chain, pathogens (including bacteria, viruses and parasites) and hazards (such as pollutants, toxins, drug residues etc.) are major contaminants of concern. In 2000, China’s Ministry of Agriculture (MOA) mandated a nationwide quality standard that awards a green label to qualified safer seafood (MOA, Fishery Administrative, Year 2000, Document #17). Since then, R&D for seafood security has been undertaken. Traceability systems, including tracking and tracing approaches, have been gradually established and improved [92, 93]. According to China’s 2010 government statistics, 787 seafood brands were granted a green label, to ensure the safety of food in China. In actuality, there is still a long way to go to guarantee the safety of seafood for consumers.
Environmental biotechnology can and should play an important role in addressing challenges related to the marine environment, as well as the threat of pollution of the aquatic products in it. Although great advances have been made in the detection and analysis of trace pollutants during recent decades, due to the continued development and refinement of specific techniques, a wide array of undetected contaminants of emerging environmental concern must be identified and quantified in various environmental components and biological tissues [94].
3.5. Bioprocess and Biorefinery
Marine biotechnology is expected to affect many value-added sectors: pharmaceuticals, food, industrial processing, nutraceuticals, etc. Tools based on marine biotechnology can be widely used in industrial processing and manufacturing and can play an important role in global green growth efforts [12]. For example, in addition to their fuel potential, algae and other marine biomass represent largely untapped alternatives to so-called platform chemicals and even functional food products. From 2003 to 2012, China’s seafood processing industry grew at an annual rate at 10.7%, twice that of its aquaculture sector [95]. China, the world’s leading aquaculture producer, has a huge annual production biomass. In addition to the material for direct consumption, there is much waste. Use of aquaculture wastes provides an important opportunity for meeting domestic fishmeal and oil demands, reducing use of trash fish in feeds, and minimizing waste discharges and pollution from processing plants. Cao L. et al. [95] estimated the potential of fishmeal and oil production from China’s fish processing industry.
To convert the huge aquatic biomass into value-added chemicals and fuels, biotechnology and nano-technologies are becoming accepted as important players, along with conventional biomass refinery technologies [96]. The objective of a biorefinery is to utilize biomass ingredients efficiently, similar to petroleum refineries in which oil is fractionated into fuels and a variety of products with higher value. Microalgae are a potential source for various valuable chemicals for commercial applications ranging from nutraceuticals to fuels. The identification and application of novel, marine-derived pharmaceuticals, cosmetics, nutritional supplements, enzymes, and pigments have already been realized.
The seaweed resources in China are very rich, with an annual output of 7.9 million tonnes from aquaculture alone (FAO 2011). Seaweeds supply not only a lot of good seafood directly for humans, but also an abundance of materials for value-added substances, such as different phycocolloids used to produce algin, agar and carrageenan, iodine and mannito. In China, the annual seaweed industry production of algin, agar, carrageenan, iodine and mannito is 20,000t, 1000t, 7500t, 180t and 4000t respectively [97]. Also some drugs (PSS and Shenhaikang) for treating cardiovascular and kidney disease are produced from seaweed.
Until 2008, there were up to 684 varieties of marine materia medica and potential medicinal resources investigated by modern pharmacology and chemistry researchers including plant medica, animal medica and mineral medica of 205, 468 and 11 varieties, respectively [98]. Novel peptides from sponges, ascidians, molluscs, sea anemones and seaweeds are presented in association with their pharmacological properties and acquisition methods. More recent studies have indicated that halogenated natural products are actually common in some sources. In fact, more than 5000 halogenated natural products had been discovered as of 2011 [99].
Conclusion
The marine biotechnological fields in China have scored unprecedented achievements under the joint efforts of the scientific research technical staff during the past decades. The blue economy is emerging at a critical juncture of the world economic transformation during the global recession. Obviously, marine biotechnology R&D is vital for advancing sectors of the blue bioeconomy. Innovative achievements in China are not enough and the level and frequency of academic achievements must be improved. More technology and products with independent intellectual property rights are needed.
Last but not least, international cooperation and assistance remain crucial for the success of marine biotechnology. China has always attached great importance to the academic exchange and cooperation with the international science and technology communities and, following the country's opening to the outside world after 1979, it has established channels for scientific and technological cooperation and exchange with many well-known universities, institutions and hi-tech enterprises globally.
There is no doubt that international cooperation, from private enterprises to government, has become increasingly prevalent. In light of the growing global economy, such cooperation is beneficial for almost every country in the world. China fully participates in the activities of relevant organizations, for example, FAO, UNEP, EU Framework Programme for Research, the ASEM (Asia-Europe Meeting) Aquaculture, World Aquaculture Society, International Marine Biotechnology Association and so on.
Fig. (1).
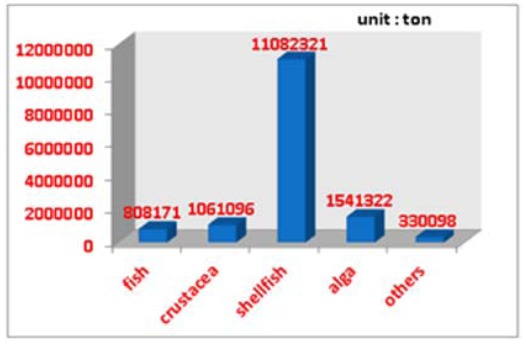
Mariculture production of China in 2010.
Fig. (2).
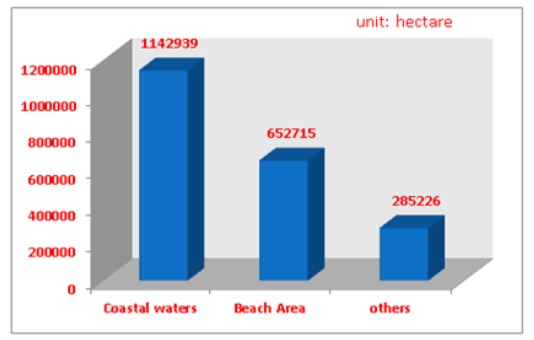
Area and location of Chinese mariculture in 2010.
Fig. (3).
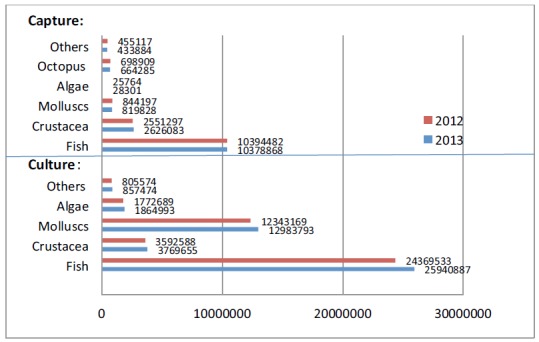
Yields of various Chinese captured and cultured aquatic products in 2012 and 2013. (unit: Tonnes).
Table 1.
Major New Varieties in Chinese Aquaculture Approved by NCCAPV Since 2004.
| Species | Novel Variety |
Appr.
Year |
Innovatotrs | Produced Methods | Major Merits |
|---|---|---|---|---|---|
|
Laminaria japonica (Sea Kelp) |
Rongfu Haidai | 2004 | OUC et al. | Hybridization & selection |
GR(W): +25-27% Tolerate higher temperature |
|
Laminaria japonica (Sea Kelp) |
Dongfang 2 | 2004 | Shandong DFMST Ltd. |
Hybridization | GR(W): +28-59% |
|
Laminaria japonica (Sea Kelp) |
Huangguan 1 | 2011 | YSFRI et al. | Hybridization & mass selection |
GR(W): + >27% |
|
Laminaria japonica (Sea Kelp) |
Sanhai Haidai | 2012 | OUC et al. | Hybridization | GR(W): +11.1%, with longitudinal furrow on leaf |
|
Laminaria japonica (Sea Kelp) |
Dongfang 6 | 2013 | Shandong DFMST Ltd |
Hybridization of two clones of gametophyte |
GR(W): + 36-46% |
| Gracilaria lemaneiformis | Longxucai 2007 | 2013 | OUC & Shantuo University |
Metagenic & mass selection |
GR(W): +17.7% Higher agar-agar |
| Undaria pinnatifida | Haibao 1 | 2013 | IOCAS & Dalian Haibao Fishery Ltd |
Crossing haploids & selection |
GR(W): +48.1%, |
|
Porphyra haitanensis (Nori) |
SHENFU 1 | 2009 | SOU | Metagenesis & cell cloning breeding |
GR(W): +25%,better quality |
| Porphyra haitanensis (Nori) | SHENFU 2 | 2013 | SOU, et al. | Metagenesis & cell cloning breeding |
GR(W): +28-35% better quality,higherfertility +43% |
|
Porphyra haitanensis (Nori) |
Minfeng 1 | 2012 | Jimei University | Metagenesis & clone breeding |
GR(W): + >25%, Survivable at 28°C for ten days |
| Porphyra yezoensis | Sutong 1 | 2013 | JSMFRI | Metagenesis & mass selection |
GR(W): +37.8%, Higher Protein & UFA |
|
Meretrix meretrix (Clam) |
Kezhe 1 | 2013 | IOCAS & ZJIMC | Mass & family selection |
GR(W): +31.6%, with black strip |
| Ostrea gigas (Oyster) | Haida 1 | 2013 | OUC | Mass selection | GR(W): + 18-24% |
|
Patinopecten yessoensis
(Scallop) |
Haida Jingbei | 2009 | OUC, & Zhangzi Fishery Ltd. |
Mass & family selection | GR(W):+20-30%, SR:+25% Higher carotene |
|
Argopecten irradias (Bay scallop) |
Zhongke 2 | 2011 | IOCAS | Family selection with Inbreeding & hybridization |
GR(W): +26.57% Purple shell |
|
Pinctada martensi (Pearl scallop) |
Haiyou 1 | 2011 | Hainan University | Hybridization & mass selection |
GR(W):+24.9% Pearl beads rate: +15.90%, |
|
Chlamys farreri (Scallop) |
Penglai Red 2 | 2013 | OUC et al. | Family selection | GR(W): +53.46%, SR: +27.11% |
| Hybrids of Abalone Haliotis discus hanai | Dalian 1 | 2004 | IOCAS | Hybridization | GR(W): + >20%, SR: + 180-230% |
|
Haliotis diversicolor (Abalone) |
Dongyou 1 | 2009 | Xiamen University | Hybridization | GR(W):+35%, SR: +35% |
|
Fenneropenaeus chinensis
(Shrimp) |
Huanghai 1 | 2004 | YSFRI | Mass selection | GR(W):+26.9% |
|
Fenneropenaeus chinensis
(Shrimp) |
Huanghai 2 | 2008 | YSFRI | Mass and family selection |
GR(W):+30% SR: +12.25-20.7% |
|
Fenneropenaeus chinensis
(Shrimp) |
Huanghai 3 | 2013 | YSFRI | Mass selection | GR(W):+11.8% SR: +11.8% |
|
Litopenaeus vannamei
(Shrimp) |
Kehai 1 | 2010 | IOCAS et al. | Family + mass selection |
GR(W):+12.6-41.7% (depend on density) |
|
Litopenaeus vann amei (Shrimp) |
Zhongxin 1 | 2010 | Sun Yat-Sen Uni. & Hengxin Ltd. |
Family selection | SR(W): +20% |
|
Litopenaeus vannamei
(Shrimp) |
Zhongke 1 | 2010 | SCSIOCAS et al. | Mass & family selection |
GR(W): +21.8% |
| Species | Novel Variety |
Appr. Year |
Innovatotrs | Produced Methods | Major Merits |
|
Litopenaeus vannamei
(Shrimp) |
Guihai 1 | 2012 | Guangxi FRI | Family selection | GR(W): +13.97-15% SR: + 11.32% |
|
Penaeus monodon (Shrimp) |
Nanhai 1 | 2010 | SCSFRI | Mass selection | GR(W): +21.6%-24.4% SR: +8.4% |
|
Macrobrachium rosenbergii
(Prawn) |
Taihu 1 | 2008 | ZJFWFRI | Hybridization & mass selsction |
GR(W): +36.87%, SR: +7.76% |
|
Eriocheir sinensis (River Crab) |
Guanghe 1 | 2011 | Panjing Guangghe Crab Farm Ltd. |
Mass selection | GR(W): +25.98% SR: +48.59% |
|
Eriocheir sinensis (River Crab) |
Changjiang 1 | 2011 | JSFWFRI | Mass selection | GR(W): +16.7% Better uniformity |
|
Eriocheir sinensis (River Crab) |
Changjiang 2 | 2013 | JSFWFRI | Mass selection | GR(W): +18.5% Better uniformity |
| Portunus trituberculatus | Huangxuan 1 | 2012 | YSFRI et al. | Mass selection | GR(W): +20.12% SR: +30% |
| Portunus trituberculatus | Keyong 1 | 2013 | IOCAS & Ningbo Uni. |
Mass selection | GR(W): +11.4%, SR: +13.9% |
|
Apostichopus japonica (Sea Cucumber) |
Shuiyuan 1 | 2009 | DOU et al. | Hybridization & selection |
GR(W): +30% |
| Hybrids of Carp (♀) and Crucian (♂) |
Furong Li-Ji | 2009 | Hunan FSI | Hybridization | 7.8 times bigger than the ♂parent about 86.2% of the ♀parent. |
| Triplods of Crucian | Xiangyun Ji 2 | 2008 | Hunan Normal Uni. |
Hybridization & cyto- engineering |
Higher growth rate (1.43-4.21 times) and better taste |
| Hybrids of Tilapia (Oreochromis spp.) |
Jili Tilapia | 2009 | SOU, et al. | Hybridization | Cultured in S 15~25‰water, grow up to 500g in 5-6 monthes |
| Hybrids of Snakehead (Channa maculate x Ophicephalus argus) |
Hang Li 1 | 2009 | Hangzhou ASA | Hybridization | GR(W): +20%-50% 0.5~0.7kg/ind. at 1 Yr age |
|
Xenocypris argentea X Megalobrama amblycephala |
Bian-Gu Hybrids | 2011 | Hunan Normal Uni. |
Hybridization | GR(W): +11.67-37.5% Infertility |
| Hybrids of red carp and mirror carp |
Songbu Red Mirror Carp | 2008 | HLJFRI | Hybridization + mass selection |
GR(w): +21.6-35.6% SR: +12.2-12.9%. |
| Hybrids of Carassius auratus
(Crucian) |
Jin Xin Wuji | 2013 | Tianjin Huanxin Breeding Farm |
Hybridization & cyto engineering |
GR(W): +10-16% |
|
Paralichthys olivaceus (Flatfish) |
Pingyou 1 | 2010 | YSFRI et al. | GR(W): +30%, SR: +20% |
|
|
Scophthalmus maximus
(Turbot) |
Danfa Ping | 2010 | YSFRI et al. | Hybridization & mass selection |
GR(w): +24% SR: +18%. |
|
Larimichthys crocea
(Large yellow croaker) |
Mingyou 1 | 2010 | Jimei Uni. et al. | Hybridization of 3 populations & selection |
GR(w): +23.9%, SR: +13.7%. |
|
Larimichthys crocea
(Large yellow croaker) |
Donghai 1 | 2013 | Ningbo Uni. et al. | Mass selection | GR(w): +15.57%, SR (at low temp): +22.5%. |
|
Ictalurus punctatu (Catfish) |
Jiang Feng 1 | 2013 | JSFFRI et al. | Hybridization | GR(W) +22.1%-25.3% |
|
Micropterus salmoides (Perch) |
Youlu 1 | 2010 | PRFRI et al. | Mass selection | GR(W):+17.8-25.3% |
|
Cyprinus carpio carpio
(Carp) |
Furui Li | 2010 | FWFRC | Mass & family selection |
GR(W):+ >20% |
|
Hypophthalmi- chthys molitrix (Silver carp) |
Changfeng Lian | 2010 | YRFRI | Gynogenesis & mass selection |
GR(W): +13.3-20.5% (depend on age) |
|
Hypophthalmi- chthys molitrix |
Jin Lian | 2010 | Tianjin Huanxin Breeding Farm |
Mass selection | GR(W): +10-13.2% RR:+74% |
| Species | Novel Variety |
Appr. Year |
Innovatotrs | Produced Methods | Major Merits |
| Oujiang Color carp | Longshen 1 | 2011 | SOU et al. | Mass selection | GR(W): +13.68-24.65%. |
| All female of (Flatfish) Paralichthys olivaceus |
Beiping 1 | 2011 | BDHCES | Hybridization of 2 gynogenesis families |
90% female GR(W): +15.59-23.37% |
| All female of (Flatfish) Paralichthys olivaceus |
Beiping 2 | 2013 | BDHCES et al. | Gynogenesis & sex-reversion |
90% female GR(W): +50% |
| All male of Tilapia Oreochromis niloticus |
Luxiong 1 | 2012 | Xiamen Luye Fishery Ltd. |
Cyto-engineering | Male rate: >99% |
| All male of yellow catfish Pelteobagrus fulvidraco |
Quanxiong 1 | 2010 | IWEECAS et al. | Cyto-engineering | All male GR(W): +43.5-56.8% |
| Allogynogenetic silver crucian carp |
Zhongke 3 | 2008 | IHBCAS | Allogyno-genesis | GR(W): + 13.7-34.4% |
Source: Announcements related to Aquaculture Protospecies and Varieties by
the Ministry of Agriculture of China from 2004 to 2014.
OUC: Oceanic University of China;
DFMST Ltd: Dongfang Marine Science & Technology Ltd;
IOCAS: Institute of Oceanology, Chinese Academy of Sciences;
JSMFRI: Jiangsu Marine Fisheries Research Institute;
ZJIMC: Zhejiang Institute of Mariculture;
SCSIOCAS: South China Sea Institute of Oceanology, Chinese Academy of Sciences;
Guangxi FRI: Guangxi Fisheries Research Institute;
SCSFRI: South China Sea Fisheries Research Institute, Chinese Fisheries Research Academy;
ZJFWFRI:Zhejiang Freshwater Fisheries Research Institute;
JSFWFRI:Jiangsu Freshwater Fisheries Research Institute;
Hangzhou ASA:Hangzhou Agriculture Science Academy;
DOU Dalian Oceanic University;
HLJFRI:Heilongjiang Fisheries Research Institute, Chinese Fisheries Research Academy;
YSFRI:Yellow Sea Fisheries Research Institute,Chinese Fisheries Research Academy;
JSFFRI: Jiangsu Freshwater Fisheries Research Institute;
PRFRI: Pearl River Fisheries Research Institute, Chinese Fisheries Research Academy;
FWFRC: Freshwater Fisheries Research Center, Chinese Fisheries Research Academy;
YRFRI: Yangtze River Fisheries Research Institute, Chinese Fisheries Research Academy;
SOU: Shanghai Oceanic University;
BDHCES, CFRA: BeiDaiHe Experimental Center Station;
IWEECAS: Institute of Water Ecology and Engineering, Chinese Academy of Sciences;
IHBCAS: Institute of Hydrobiology, Chinese Academy of Sciences.
ACKNOWLEDGEMENTS
Special thanks to Prof. Yonathan Zohar and John Stubblefield from the University of Maryland for their useful advice and for carefully editing to my manuscript. I am grateful to Prof. Ying Liu for his generous permission to use his pictures as Figs. (5-7) in this paper. My research has been supported by the National High Technology Research and Development Program (“863” Program, 2012AA10A404) of China and the Major State Basic Research Development Programs of China (“973” Program, 2012CB114403) sponsored by Ministry of Science and Technology of China.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Wei X.F., Xu J. Comparative Study on International and Chinese Agriculture. Agric. Econ. 2000;9:45–47. [Google Scholar]
- 2.Xiang J. Marine Science & Technology in China . A Roadmap to 2050. Heidelberg: Springer Verlag GmbH; 2010. [Google Scholar]
- 3.FAO The State of world fisheries and aquaculture-2010. 2012.
- 4.Zeng C.K. Some remarks on marine biotechnology in China. Current Topics in Marine Biotechnology. Tokyo, Japan: The Japannese Society for Marine Biotechnology; 1989. [Google Scholar]
- 5.FAO State of world aquaculture 2006. Rome. FAO Fish. Tech. Pap. 2006:500. [Google Scholar]
- 6.Fishery Bureau of the Ministry of Aqriculture of China . China Fishery Statistical Yearbook. Beijing: The Publish House of Chinese Aqriculture; 2014. p. 145. [Google Scholar]
- 7.State Oceanic Administration of China 2015.
- 8.Xiang J. The marine biotechnology enable development of the blue bioeconomy. Chin Bull Life Sci. 2012;24(9):967–979. [Google Scholar]
- 9.Gui J.F. Fish biology and biotechnology is the source for sustainable aquaculture Special Topic: Fish biology and biotechnology. Sci. China Life Sci. 2015;58(2):121–123. doi: 10.1007/s11427-015-4812-9. [DOI] [PubMed] [Google Scholar]
- 10.Subasinghe R.P., Arthur J.R., Bartley D.M., et al. Farming the Waters for People and Food.; 2012. [Google Scholar]
- 11.Cressey D. Aquaculture: Future fish. Nature. 2009;458:398–400. doi: 10.1038/458398a. [DOI] [PubMed] [Google Scholar]
- 12.OECD . Marine Biotechnology: Enabling Solutions for Ocean Productivity and Sustainability. Paris: OECD Publishing; 2013. [Google Scholar]
- 13.Zeng C.K., Xiang J.H. Marine Biotechnology. Jinan: Publish House of Shandong Science and Technology; 1998. [Google Scholar]
- 14.Sun H. Overview of development of the marine high technology in China. Chinese & Foreign Corporate Culture. 2001;12:54–59. [Google Scholar]
- 15.Zohar Y., Munoz-Cueto J.A., Elizur A., Kah O. Neuroendocrinology of reproduction in teleost fish. Gen. Comp. Endocrinol. 2010;165(3):438–455. doi: 10.1016/j.ygcen.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Zohar Y., Mylonas C.C. Endocrine manipulations of spawning in culturedfish: from hormones to genes. Aquaculture. 2001;197(1-4):99–136. [Google Scholar]
- 17.Gui J.F., Zhu Z.Y. Molecular basis and genetic improvement of economically important traits in aquaculture animals. Chin. Sci. Bull. 2012;57(15):1751–1760. [Google Scholar]
- 18.Xu K., Duan W., Xiao J., et al. Development and application of biological technologies in fish genetic breeding. Sci. China Life Sci. 2015;58(2):187–201. doi: 10.1007/s11427-015-4798-3. [DOI] [PubMed] [Google Scholar]
- 19.Gjedrem T., Baranski M. Selective breeding in Aquaculture: An Introduction. New York: Springer; 2009. [Google Scholar]
- 20.Liu Z.J., Cordes J.F. DNA marker technologies and their applications in aquaculture genetics. Aquaculture. 2004;238(1-4):1–37. [Google Scholar]
- 21.Xiong F., Wei Z.Q., Zhu Z.Y., Sun Y.H. Targeted expression in zebrafish primordial germ cells by Cre/loxP and Gal4/UAS systems. Mar. Biotechnol. (NY) 2013;15(5):526–539. doi: 10.1007/s10126-013-9505-4. [DOI] [PubMed] [Google Scholar]
- 22.Sellars M.J., Li F., Preston N.P., Xiang J. Penaeid shrimp polyploidy: Global status and future direction. Aquaculture. 2010;310(1-2):1–7. [Google Scholar]
- 23.Tang J., Zhang F., Chen Z. Achievement and development trend of genetics and breeding of mariculture varieties in China. South China Fish Sci. 2009;5(4):77–84. [Google Scholar]
- 24.Li M.S., Lin L.S., Zhao L. Analysis of development status, trends and countermeasures of aquatic seed industry in China. Chin Fish Econ. 2013;31:139–145. [Google Scholar]
- 25.Zhu Z.Y., Li G., He L., Chen S. Novel gene transfer into the fertilized eggs of gold fish (Carassius auratus L. 1758). J. Appl. Ichthyology. 1985;1(1):31–34. [Google Scholar]
- 26.Ye D., Zhu Z.Y., Sun Y.H. Fish genome manipulation and directional breeding. Sci. China Life Sci. 2015;58(2):170–177. doi: 10.1007/s11427-015-4806-7. [DOI] [PubMed] [Google Scholar]
- 27.Hou R., Bao Z.M., Wang S., et al. Transcriptome Sequencing and De Novo Analysis for Yesso Scallop (Patinopecten yessoensis) Using 454 GS FLX. PLoS One. 2011;6(6):e21560. doi: 10.1371/journal.pone.0021560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z.J. Aquaculture Genome Technologies.Wiley-Blackwell; 2007. [Google Scholar]
- 29.Qin S., Watabe S., Lin H. Omics in marine biotechnology. Chin. Sci. Bull. 2012;57(25):3251–3252. [Google Scholar]
- 30.Xie C.T., Li B., Xu Y., Ji D.H., Chen C.S. Characterization of the global transcriptome for Pyropia haitanensis (Bangiales, Rhodophyta) and development of cSSR markers. BMC Genomics. 2013;14:107. doi: 10.1186/1471-2164-14-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Querellou J, Børresen T, Boyen C, et al. Marine Biotechnology: A new vision and strategy for Europe (Marine Board-ESF Position Paper 15). 2010.
- 32.Huete-Perez J.A., Quezada F. Genomic approaches in marine biodiversity and aquaculture. Biol. Res. 2013;46(4):353–361. doi: 10.4067/S0716-97602013000400007. [DOI] [PubMed] [Google Scholar]
- 33.Savolainen O., Lascoux M., Merila J. Ecological genomics of local adaptation. Nat. Rev. Genet. 2013;14(11):807–820. doi: 10.1038/nrg3522. [DOI] [PubMed] [Google Scholar]
- 34.Yang F., He J., Lin X.H., et al. Complete genome sequence of the shrimp white spot bacilliform virus. J. Virol. 2001;75(23):11811–11820. doi: 10.1128/JVI.75.23.11811-11820.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He J.G., Deng M., Weng S.P., et al. Complete genome analysis of the mandarin fish infectious spleen and kidney necrosis iridovirus. Virology. 2001;291(1):126–139. doi: 10.1006/viro.2001.1208. [DOI] [PubMed] [Google Scholar]
- 36.Huang X., Huang Y., Xu L., et al. Identification and characterization of a novel lymphocystis disease virus isolate from cultured grouper in China. J. Fish Dis. 2015;38(4):379–387. doi: 10.1111/jfd.12244. [DOI] [PubMed] [Google Scholar]
- 37.Lei X.Y., Ou T., Zhu R.L., Zhang Q.Y. Sequencing and analysis of the complete genome of Rana grylio virus (RGV). Arch. Virol. 2012;157(8):1559–1564. doi: 10.1007/s00705-012-1316-9. [DOI] [PubMed] [Google Scholar]
- 38.Shi C.Y., Jia K.T., Yang B., Huang J. Complete genome sequence of a Megalocytivirus (family Iridoviridae) associated with turbot mortality in China. Virol. J. 2010;7:159. doi: 10.1186/1743-422X-7-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai C.T., Ting J.W., Wu M.H., Wu M.F., Guo I.C., Chang C.Y. Complete genome sequence of the grouper iridovirus and comparison of genomic organization with those of other iridoviruses. J. Virol. 2005;79(4):2010–2023. doi: 10.1128/JVI.79.4.2010-2023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang G.F., Fang X.D., Guo X.M., et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature. 2012;490(7418):49–54. doi: 10.1038/nature11413. [DOI] [PubMed] [Google Scholar]
- 41.Xu P., Zhang X.F., Wang X.M., et al. Genome sequence and genetic diversity of the common carp, Cyprinus carpio. Nat. Genet. 2014;46(11):1212–1219. doi: 10.1038/ng.3098. [DOI] [PubMed] [Google Scholar]
- 42.Chen S.L., Zhang G.J., Shao C.W., et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat. Genet. 2014;46(3):253–262. doi: 10.1038/ng.2890. [DOI] [PubMed] [Google Scholar]
- 43.Wu C.W., Zhang D., Kan M.Y., et al. The draft genome of the large yellow croaker reveals well-developed innate immunity. Nat. Commun. 2014:5. doi: 10.1038/ncomms6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ao J.Q., Mu Y.N., Xiang L.X., et al. Genome Sequencing of the Perciform Fish Larimichthys crocea Provides Insights into Molecular and Genetic Mechanisms of Stress Adaptation. PLoS Genet. 2015;11(4):e1005118–e1005118. doi: 10.1371/journal.pgen.1005118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye N.H., Zhang X.W., Miao M., et al. Saccharina genomes provide novel insight into kelp biology. Nat. Commun. 2015:6. doi: 10.1038/ncomms7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y.P., Lu Y., Zhang Y., et al. The draft genome of the grass carp (Ctenopharyngodon idellus) provides insights into its evolution and vegetarian adaptation. Nat. Genet. 2015;47(6):625–631. doi: 10.1038/ng.3280. [DOI] [PubMed] [Google Scholar]
- 47.Chang Y., Sun X. Advancements in Genetic Linkage Maps and Quantitative Trait Locations of Aquatic Farming Animals. Zool Res. 2006;27(5):533–540. [Google Scholar]
- 48.Zhang L.S., Yang C.J., Zhang Y., et al. A genetic linkage map of Pacific white shrimp (Litopenaeus vannamei): sex-linked microsatellite markers and high recombination rates. Genetica. 2007;131(1):37–49. doi: 10.1007/s10709-006-9111-8. [DOI] [PubMed] [Google Scholar]
- 49.Liu B., Wang Q.Y., Li J.A., Liu P., He Y.Y. A genetic linkage map of marine shrimp Penaeus (Fenneropenaeus) chinensis based on AFLP, SSR, and RAPD markers. Chin. J. Oceanology Limnol. 2010;28(4):815–825. [Google Scholar]
- 50.Liu L., Li J., Liu P., Zhao F.Z., Gao B.Q., Du Y. Identification of quantitative trait loci for growth-related traits in the swimming crab Portunus trituberculatus. Aquacult. Res. 2015;46(4):850–860. [Google Scholar]
- 51.Li L., Xiang J.H., Liu X., Zhang Y., Dong B., Zhang X.J. Construction of AFLP-based genetic linkage map for Zhikong scallop, Chlamys farreri Jones et Preston and mapping of sex-linked markers. Aquaculture. 2005;245(1-4):63–73. [Google Scholar]
- 52.Yuan T., He M.X., Huang L.M., Hu J.X. Genetic Linkage Maps of the Noble Scallop Chlamys Nobilis Reeve Based on Aflp and Microsatellite Markers. J. Shellfish Res. 2010;29(1):55–62. [Google Scholar]
- 53.Wang Y.P., Guo X.M. Development and characterization of EST-SSR markers in the eastern oyster Crassostrea virginica. Mar. Biotechnol. (NY) 2007;9(4):500–511. doi: 10.1007/s10126-007-9011-7. [DOI] [PubMed] [Google Scholar]
- 54.Liu X.D., Liu X., Guo X.M., Gao Q.K., Zhao H.G., Zhang G.F. A preliminary genetic linkage map of the pacific abalone Haliotis discus hannai Ino. Mar. Biotechnol. (NY) 2006;8(4):386–397. doi: 10.1007/s10126-005-6133-7. [DOI] [PubMed] [Google Scholar]
- 55.Yan J.J., Jing J., Mu X.Y., et al. A genetic linkage map of the sea cucumber (Apostichopus japonicus) based on microsatellites and SNPs. Aquaculture. 2013;404:1–7. [Google Scholar]
- 56.Zhou Z.C., Bao Z.M., Dong Y., et al. AFLP linkage map of sea urchin constructed using an interspecific cross between Strongylocentrotus nudus (female) and S-intermedius (male). Aquaculture. 2006;259(1-4):56–65. [Google Scholar]
- 57.Ye H., Liu Y., Liu X.D., Wang X.Q., Wang Z.Y. Genetic Mapping and QTL Analysis of Growth Traits in the Large Yellow Croaker Larimichthys crocea. Mar. Biotechnol. (NY) 2014;16(6):729–738. doi: 10.1007/s10126-014-9590-z. [DOI] [PubMed] [Google Scholar]
- 58.Liu Q., Sakamoto T., Kubota S., et al. A genetic linkage map of kelp grouper (Epinephelus bruneus) based on microsatellite markers. Aquaculture. 2013;414:63–81. [Google Scholar]
- 59.Song W.T., Pang R.Y., Niu Y.Z., et al. Construction of High-Density Genetic Linkage Maps and Mapping of Growth-Related Quantitative Trail Loci in the Japanese Flounder (Paralichthys olivaceus). PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0050404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song W.T., Li Y.Z., Zhao Y.W., et al. Construction of a High-Density Microsatellite Genetic Linkage Map and Mapping of Sexual and Growth-Related Traits in Half-Smooth Tongue Sole (Cynoglossus semilaevis). PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0052097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang X.J., Zhang Y., Scheuring C., et al. Construction and Characterization of a Bacterial Artificial Chromosome (BAC) Library of Pacific White Shrimp, Litopenaeus vannamei. Mar. Biotechnol. (NY) 2010;12(2):141–149. doi: 10.1007/s10126-009-9209-y. [DOI] [PubMed] [Google Scholar]
- 62.Yu Y, Zhang XJ, Yuan JB, et al. Genome survey and high-density genetic map construction provide genomic and genetic resources for Pacific White Shrimp Litopenaeus vannamei. 2015. [DOI] [PMC free article] [PubMed]
- 63.Jiang Z.J. Environmental effect of marine fish cage aquaculture and integrated multi-trophic aquaculture. Environmental Science and Management. 2012;37(1):120–125. [Google Scholar]
- 64.Troell M., Joyce A., Chopin T., Neori A., Buschmann A.H., Fang J.G. Ecological engineering in aquaculture - Potential for integrated multi-trophic aquaculture (IMTA) in marine offshore systems. Aquaculture. 2009;297(1-4):1–9. [Google Scholar]
- 65.Wu H.L., Huo Y.Z., Han F., Liu Y.Y., He P.M. Bioremediation using Gracilaria chouae co-cultured with Sparus macrocephalus to manage the nitrogen and phosphorous balance in an IMTA system in Xiangshan Bay, China. Mar. Pollut. Bull. 2015;91(1):272–279. doi: 10.1016/j.marpolbul.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Y., Yang H.S., Hu H.Y., et al. Bioremediation potential of the macroalga Gracilaria lemaneiformis (Rhodophyta) integrated into fed fish culture in coastal waters of north China. Aquaculture. 2006;252(2-4):264–276. [Google Scholar]
- 67.Hargreaves JA. Biofloc production systems for aquaculture.Southern regional aquaculture center Publication. 2013.
- 68.Liang W.Y., Luo G.Z., Tan H.X., Ma N.N., Zhang N., Li L. Efficiency of biofloc technology in suspended growth reactors treating aquacultural solid under intermittent aeration. Aquacult. Eng. 2014;59:41–47. [Google Scholar]
- 69.Liu L., Hu Z., Dai X., Avnimelech Y. Effects of addition of maize starch on the yield, water quality and formation of bioflocs in an integrated shrimp culture system. Aquaculture. 2014;418:79–86. [Google Scholar]
- 70.Xu W.J., Pan L.Q., Zhao D.H., Huang J. Preliminary investigation into the contribution of bioflocs on protein nutrition of Litopenaeus vannamei fed with different dietary protein levels in zero-water exchange culture tanks. Aquaculture. 2012;350:147–153. [Google Scholar]
- 71.Guo G., Tao Q., Huang X., Hu Y. Progress on Frontier of Equipment Technology for Sea-cage Aquaculture. Rev China Agric Sci Technol. 2011;13(5):44–49. [Google Scholar]
- 72.FAO . FAO biosecurity toolkit. Rome: FAO; 2007. p. 127. [Google Scholar]
- 73.Xiang J.H., Zhao Y.F. Introduction of a special issue on “Comparative Immunology in China”. Dev. Comp. Immunol. 2013;39:1. doi: 10.1016/j.dci.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 74.Zhu L.Y., Nie L., Zhu G., Xiang L.X., Shao J.Z. Advances in research of fish immune-relevant genes: A comparative overview of innate and adaptive immunity in teleosts. Dev. Comp. Immunol. 2013;39(1-2):39–62. doi: 10.1016/j.dci.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 75.Li F.H., Xiang J.H. Signaling pathways regulating innate immune responses in shrimp. Fish Shellfish Immunol. 2013;34(4):973–980. doi: 10.1016/j.fsi.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 76.Li F.H., Xiang J.H. Recent advances in researches on the innate immunity of shrimp in China. Dev. Comp. Immunol. 2013;39(1-2):11–26. doi: 10.1016/j.dci.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 77.Guo Z.X., Zhang D., Ma H.L., Su Y.L., Feng J., Xu L.W. Rapid detection of mud crab dicistrovirus-1 using loop-mediated isothermal amplification. J. Virol. Methods. 2014;208:171–176. doi: 10.1016/j.jviromet.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 78.Li K., Clausen J.H., Murrell K.D., Liu L., Dalsgaard A. Risks for fishborne zoonotic trematodes in Tilapia production systems in Guangdong province, China. Vet. Parasitol. 2013;198(1-2):223–229. doi: 10.1016/j.vetpar.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 79.Xu L., Huang J., Yang B. Application of gene chip in pathogen detection and its prospect in aquaculture. Mar. Fish. Res. 2008;29(1):109–114. [Google Scholar]
- 80.Yang T.B., Chen A.P., Chen W., Li A.X., Yan Y.Y. Parasitic diseases of cultured marine finfishes and their surveillance in China. Parassitologia. 2007;49(3):193–199. [PubMed] [Google Scholar]
- 81.Zeng W.W., Wang Q., Shi C., Wu S. Application of Immunology and Molecular Biology Techniques in Diagnosing Diseases of Aquatic Animals. Prog Vet Med. 2010;31:111–117. [Google Scholar]
- 82.Zhang D.F., Zhang Q.Q., Li A.H. Development of a multiplex PCR assay for rapid and simultaneous detection of four genera of fish pathogenic bacteria. Lett. Appl. Microbiol. 2014;59(5):471–478. doi: 10.1111/lam.12303. [DOI] [PubMed] [Google Scholar]
- 83.Sun R., Qiu L.M., Yue F., et al. Hemocytic immune responses triggered by CpG ODNs in shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2013;34(1):38–45. doi: 10.1016/j.fsi.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 84.Xu T., Zhang X. Edwardsiella tarda: an intriguing problem in aquaculture. Aquaculture. 2014;431:129–135. [Google Scholar]
- 85.Zhang Y., Li Q., Wang Y., Zhang D. Research status and application of probiotics to mariculture. Mar. Fish. Res. 2005;26(6):83–87. [Google Scholar]
- 86.Hu Y.H., Sun L. A bivalent Vibrio harveyi DNA vaccine induces strong protection in Japanese flounder (Paralichthys olivaceus). Vaccine. 2011;29(26):4328–4333. doi: 10.1016/j.vaccine.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 87.Jia P.P., Hu Y.H., Chi H., Sun B.G., Yu W.G., Sun L. Comparative study of four flagellins of Vibrio anguillarum: Vaccine potential and adjuvanticity. Fish Shellfish Immunol. 2013;34(2):514–520. doi: 10.1016/j.fsi.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 88.Sun B.G., Dang W., Sun L., Hua Y.H. Vibrio harveyi Hsp70: Immunogenicity and application in the development of an experimental vaccine against V. harveyi and Streptococcus iniae. Aquaculture. 2014;418:144–147. [Google Scholar]
- 89.Sun Y., Hu Y.H., Liu C.S., Sun L. A Streptococcus iniae DNA vaccine delivered by a live attenuated Edwardsiella tarda via natural infection induces cross-genus protection. Lett. Appl. Microbiol. 2012;55(6):420–426. doi: 10.1111/j.1472-765X.2012.03307.x. [DOI] [PubMed] [Google Scholar]
- 90.Wang C., Hu Y.H., Sun B.G., Chi H., Li J., Sun L. Environmental isolates P1SW and V3SW as a bivalent vaccine induce effective cross-protection against Edwardsiella tarda and Vibrio anguillarum. Dis. Aquat. Organ. 2013;103(1):45–53. doi: 10.3354/dao02569. [DOI] [PubMed] [Google Scholar]
- 91.Rico A., Satapornvanit K., Haque M.M., et al. Use of chemicals and biological products in Asian aquaculture and their potential environmental risks: a critical review. Rev Aquacult. 2012;4(2):75–93. [Google Scholar]
- 92.Liu Y., Ma D.H., Wang X.C., Liu L.P., Fan Y.X., Cao J.X. Prediction of chemical composition and geographical origin traceability of Chinese export tilapia fillets products by near infrared reflectance spectroscopy. Food Sci. Technol. (Campinas.) 2015;60(2):1214–1218. [Google Scholar]
- 93.Sun B. Preliminary study on the quality safe traceability system of aquatic products. Fish Econ China. 2012;30:57–61. [Google Scholar]
- 94.Qi F., Li Q., Zhu L. Assessment Method of Marine Ecosystem Health. Mar Sci Bull. 2007;26(3):97–104. [Google Scholar]
- 95.Cao L., Naylor R., Henriksson P., et al. China's aquaculture and the world's wild fisheries. Science. 2015;347(6218):133–135. doi: 10.1126/science.1260149. [DOI] [PubMed] [Google Scholar]
- 96.Gravitis J., Abolin J. Biorefinery technologies for biomass conversion into chemicals and fuels towards zero emissions. Latv J Phys Tech Sci. 2013;50(5):29–43. [Google Scholar]
- 97.Xu ZPea Present status, problems and development tactics of seaweed industries in China. J Anhui Agric Sci. 2012;40:14961–14963. [Google Scholar]
- 98.Wang C., Shao C., Fu X., et al. Investigation on the status of marine materia medica resources and research in China. J. Ocean Univ. China. 2009;39(4):669–675. [Google Scholar]
- 99.Wang B.G., Gloer J.B., Ji N.Y., Zhao J.C. Halogenated organic molecules of Rhodomelaceae origin: Chemistry and biology. Chem. Rev. 2013;113(5):3632–3685. doi: 10.1021/cr9002215. [DOI] [PubMed] [Google Scholar]


