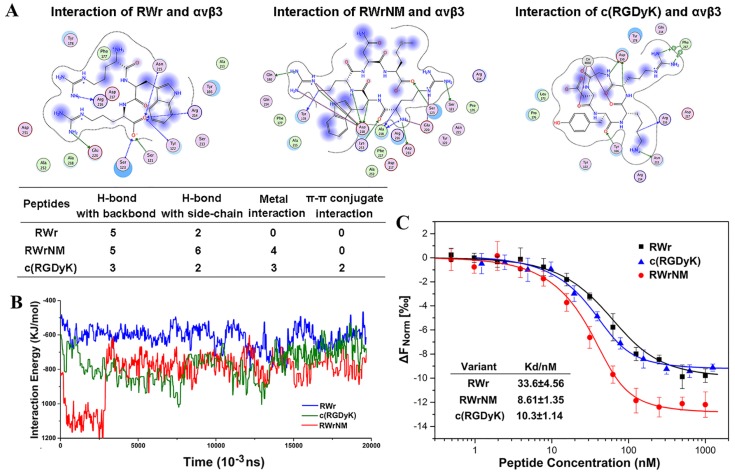
Figure 1.
Binding affinity of the peptides to αvβ3 integrin at molecular level. (A) The binding interaction mode between c(RGDyK), RWr and RWrNM peptide with αvβ3 integrin receptor after molecular docking. Residues are annotated with the 3-letter amino acid code. The active site residues are represented as follows: polar residues in light purple, hydrophobic residues in green, acidic residues with a red contour ring, basic residues with a blue contour ring. Green and blue arrows indicate hydrogen bonding to side chain and backbone atoms, respectively. The cation-π stacking interactions are represented in green dotted lines. Metal ions interaction network is represented in cyan dotted lines. Light-blue halos around residues indicate the degree of interaction with ligand atoms (larger and darker halos means more interaction). (B) Interaction energies between different peptides (RWr, c(RGDyK) and RWrNM) and αvβ3 integrin protein under 20ns. (C) The affinity of αvβ3 integrin protein to RWr, RWrNM and c(RGDyK) measured by MicroScale Thermophoresis and the Kd values of RWr, RWrNM and c(RGDyK) were calculated respectively.