Figure 4.
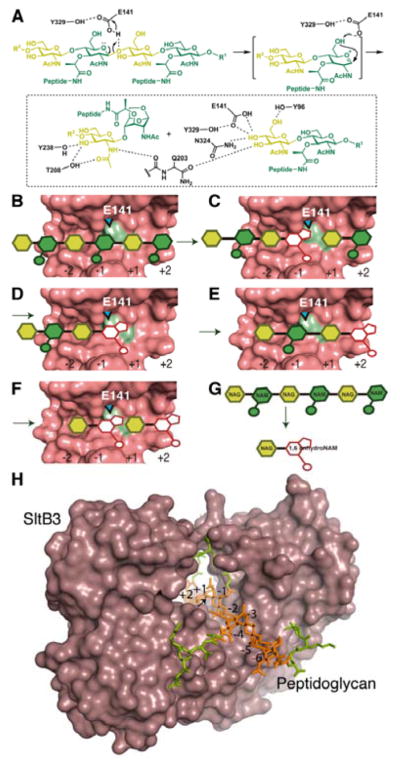
(A) The proposed catalytic scheme for SltB3. (B–F) A schematic depicting the exolytic reaction of SltB3, with the active site drawn as Connolly surface. (B) A hexasaccharide peptidoglycan binds to the active site (NAG in yellow and NAM in green). (C) The catalytic reaction produces the 1,6-anhydromuramyl moiety at the −1 subsite (in white) and a disaccharide product, which (D) departs from the active site. (E) The resulting tetrasaccharide translocates to occupy subsites −2 to +2, straddling the seat of the catalytic reaction. (F) The exolytic reaction produces two products with the 1,6-anhydromuramyl moiety. (G) The overall conversion of a hexasaccharide to the disaccharide is depicted. (H) A model of the molecular surface of SltB3 with the NMR structure of an octasaccharide peptidoglycan15 bound to the active site. The glycan chain is colored in orange and peptide stems in green. The glycosidic bond to be cleaved is indicated by an arrow.
