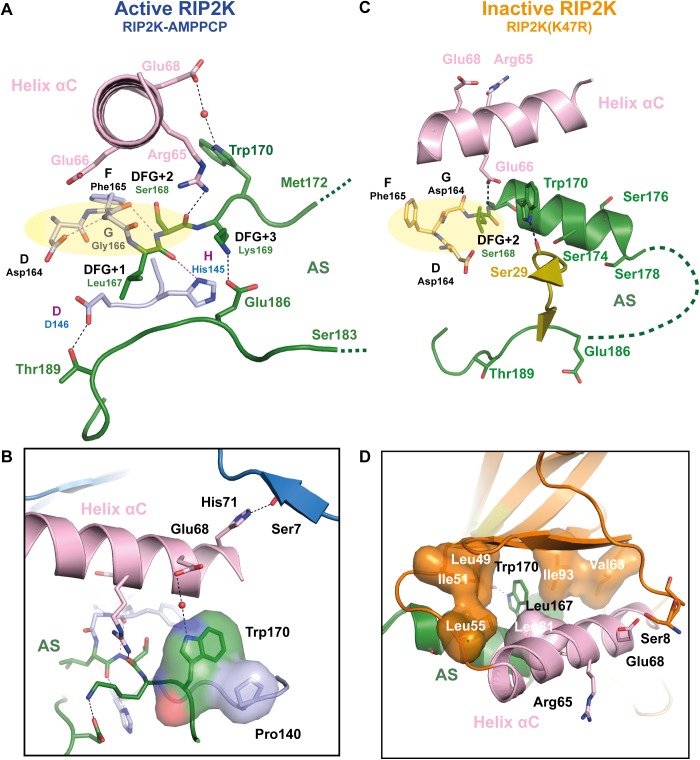
Fig 5. Helix αC, AS and kinase N-termini connections in active and inactive RIP2K.
(A-D) Detailed representation of the H-bond network between the Helix αC, AS, magnesium binding loop and N-terminus in (A-B) in RIP2K active state and (C-D) in RIP2K inactive state. For clarity, only one conformation of Ser168 side chain is shown (see S4 Fig). The insets show the interactions of Trp170 and with the N-terminus. Hydrophobic residues are shown as surface. H-bonds are represented as black dashed lines with those related to the DFG-HHD motif are in magenta. The residues belonging to the Magnesium binding loop are highlighted with a pale-yellow ellipse. Hydrophobic interactions are highlighted as surface representation. Missing AS residues are shown with a dashed line.