Figure 1. Visible light initiated thiol-norbornene reaction for forming orthogonally crosslinked biomimetic hydrogels.
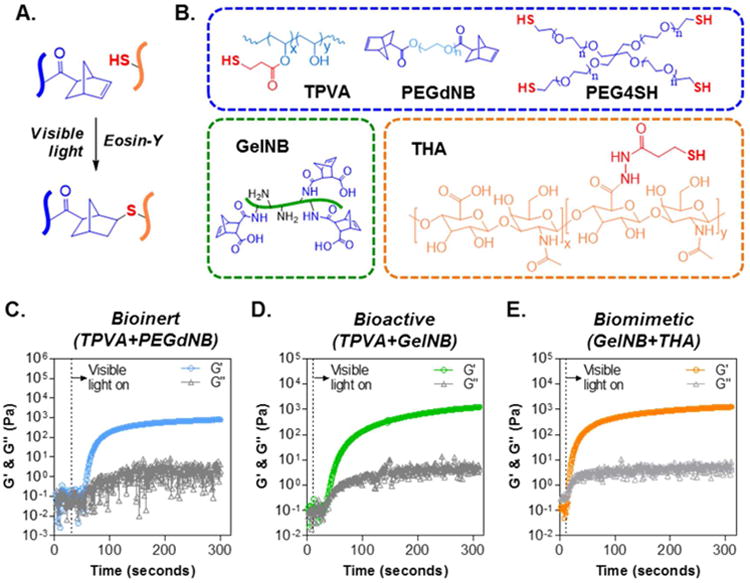
(A) Schematic of thiol-norbornene reaction under visible light exposure and with eosin-Y as the only initiator. (B) Chemical structures of macromers used, including thiolated PVA (TPVA, 6kDa), PEG-di-norbornene (PEGdNB, 10kDa), PEG-tetra-thiol (PEG4SH, 10kDa), gelatin-norbornene (GelNB), and thiolated HA (THA, 250kDa). (C-E) Evolution of storage (G′) and loss (G′) modulus of visible light initiated bio-inert (C), bioactive (D), and biomimetic (E) thiol-norbornene gelation.
