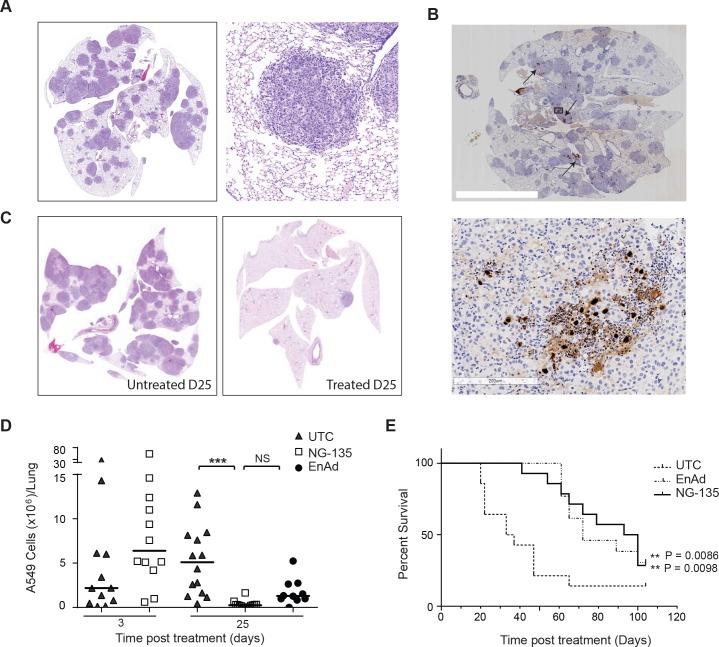
Fig 6. Antibody expression and virus activity in in vivo models following IV delivery.
(A) Representative immunohistochemical staining of mouse lungs showing disseminated lung tumour burden 8 weeks post IV delivery of human A549 lung carcinoma cells (left panel) and a zoomed image of a tumour nodule (right panel). (B) Representative IHC images of virus late gene, hexon, detection in the lungs of a mouse bearing orthotopic xenograft A549 tumour nodules, at day 25 post IV treatment with 5x109 virus particles. The left panel shows a section through the lungs, arrows indicate representative regions of hexon protein staining. The right panel shows a zoomed image of hexon protein stain within a tumour nodule. (C) Representative IHC images of tumour nodules in the lungs of mice 25 days post treatment with enadenotucirev virus particles (right panel) or mice that had not been treated by virus (left panel) (D) Quantification of total human A549 cells per lung by human cell line specific RTqPCR at days 3, 11, 18 or 25 post-IV treatment with NG-135 or enadenotucirev virus particles. Each data point represents the cell burden in a mouse lung (N>6 mice/group, ***P<0.005 one way ANOVA). (E) Kaplan-Meier plot showing percent survival for mice bearing A549 orthotopic xenograft tumours treated IV with 5x109 NG-135 or enadenotucirev virus particles, or left untreated at day 0 (n = 10–12 mice per treatment group, ** P<0.005 Log rank test).