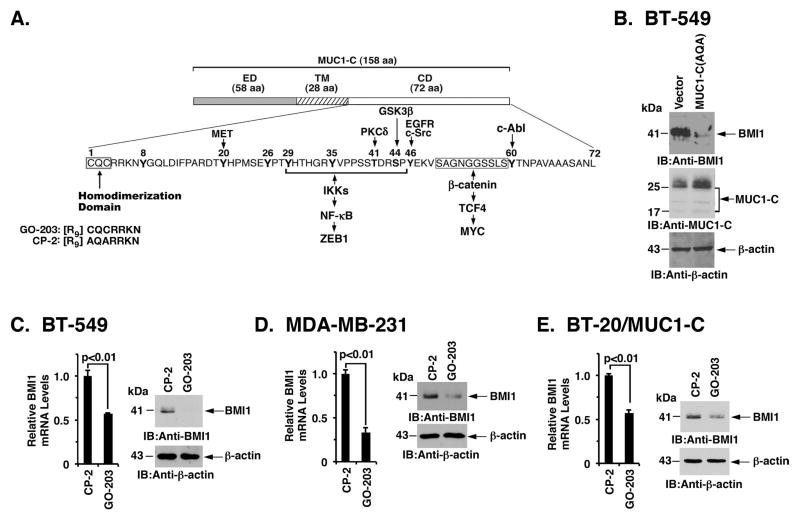
Figure 2. Targeting the MUC1-C cytoplasmic domain downregulates BMI1 expression.
A. Schema of the MUC1-C subunit with the 58 aa extracellular domain (ED), the 28 aa transmembrane domain (TM), and the sequence of the 72 aa cytoplasmic domain (CD). The MUC1-C cytoplasmic domain contains a CQC motif that is necessary and sufficient for MUC1-C homodimerization and oncogenic function. GO-203 is a cell-penetrating peptide that binds the CQC motif and blocks MUC1-C homodimerization. Highlighted are MUC1-C-induced pathways that confer the activation of ZEB1 and MYC. B. BT-549 cells were transfected with a control or MUC1-C(AQA) vector in which the CQC motif had been mutated to AQA. Lysates were immunoblotted with the indicated antibodies. C–E. BT-549 (C), MDA-MB-231 (D), and BT-20/MUC1-C (E) cells treated with 5 μM CP-2 or 5 μM GO-203 for 12 h were analyzed for BMI1 mRNA levels by qRT-PCR. The results (mean±SD) are expressed as relative BMI1 mRNA levels compared to that obtained for CP-2 (assigned a value of 1) (left). Cell lysates treated with 5 μM CP-2 or 5 μM GO-203 for 48 h were immunoblotted with the indicated antibodies (right).