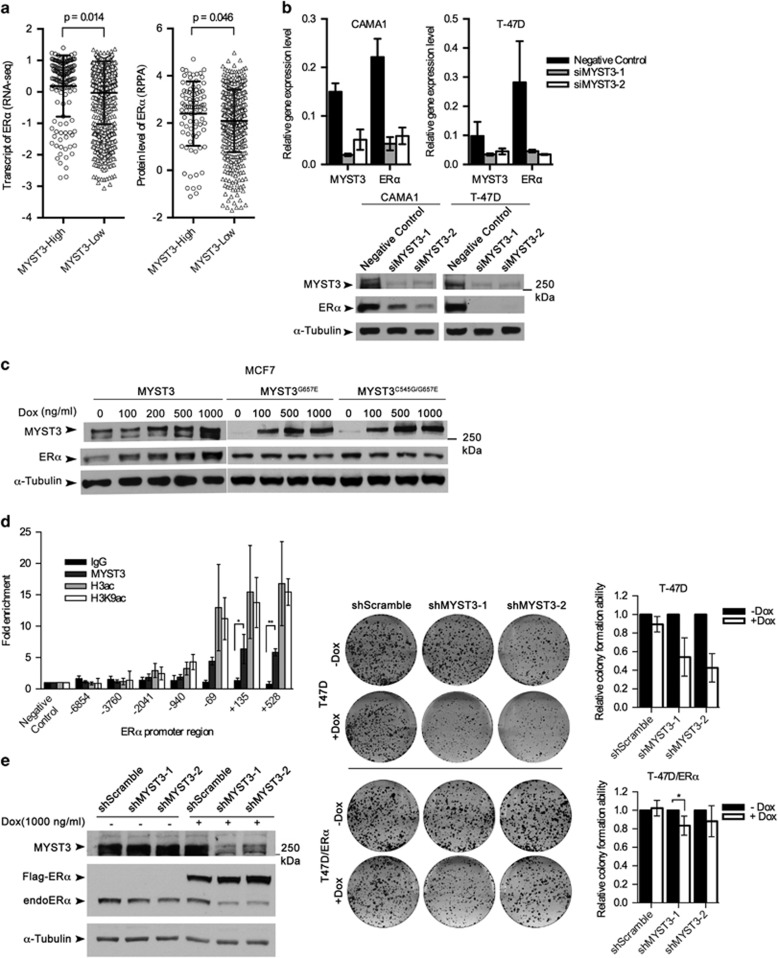
Figure 3.
MYST3 regulates ERα expression. (a) Association of ERα expression and protein level with MYST3 overexpression status in breast tumors. MYST3 expression analyses results based on TCGA RNAseq data using the cutoff of median+MAD (MAD is calculated using the R with default constant (1.4826)). MYST3-high: n=148; MYST3-low: n=954. The unit of Y-axis is transcript level of ERα (log2 of transformed upper quartile normalized read count). To estimate the correlation of ERα protein level with MYST3 expression in breast tumors, we analyzed the reverse phase protein array (RPPA) data set from TCGA. MYST3-high: n=86; MYST3-low: n=491. P-values were calculated based on student's t-test. (b) T47D and CAMA1 cells were transfected with negative control siRNA or two MYST3 siRNAs. The mRNA levels of ERα were determined with qRT–PCR (data are presented as mean±s.d, three experiments were performed), and the protein levels with western blot. Polycolonal against ERα (1:2000, Santa Cruz, #sc8002) was used to detect ERα. (c) Inducible ectopic expression of MYST3 elevated ERα levels in a dose-dependent manner. The full-length cDNA of MYST3 was purchased from Harvard plasmid deposit (Clone ID: HsCD00399202), and then was introduced into an inducible lentiviral destination vector by Gateway LR Clonase II enzyme (Life Technologies). Total protein of MCF7 cells overexpressing Flag-tagged MYST3 or its mutants were collected after a 3-day incubation with different doses of doxycycline (50–1000 ng/ml Dox). (d) T47D cells were used in the ChIP assay. ChIP was carried out by following the protocol of EZ-ChIP kit (17-371, Upstate, Temecula, CA, USA). In general, formaldehyde (final concentration: 1 %) was added to growth media to crosslink the protein to the DNA. After quenched the residual formaldehyde, cells were collected and lysed in SDS lysis buffer. Cell lysate were sonicated with three pulses of 45 s to achieve fragment sizes of around 500 bp by sonic dismembrator (Thermo Fisher Scientific). The anti-MYST3 rabbit polyclonal antibody (39867, Active Motif), anti-acetyl-Histone H3 antibody (06-599, Millipore, Darmstadt, Germany) and anti-acetyl-Histone H3(Lys9) monoclonal antibody (9649S, Cell Signaling, Danvers, MA, USA) were used to immunoprecipitate the respective crosslinked protein/DNA. qRT–PCR and primers was carried out as described23 and primer sequences are provided in Supplementary Table 1. The primers (+135, and +528) are the promoter regions of ESR1. Normal IgG was used as negative control. Data are presented as mean±s.d. Three experiments were performed. P-values were calculated based on two-tailed student's t-test. *P<0.05; **P<0.01. (e) Restoration ERα expression in stably inducible MYST3 shRNAs cells partially recovered the cell growth. The full-length cDNA of ERα was purchased from Harvard plasmid deposit (Clone ID: HsCD00376961), and then was introduced into an inducible lentiviral destination vector by Gateway LR Clonase II enzyme. Inducibly expressing ERα and MYST3 shRNAs in T47D cells were induced with doxycycline. The protein levels were determined with western blot. Statistical data of clonogenic assay are presented as mean±s.d. Five experiments were performed. P-values were calculated based on two-tailed student's t-test.