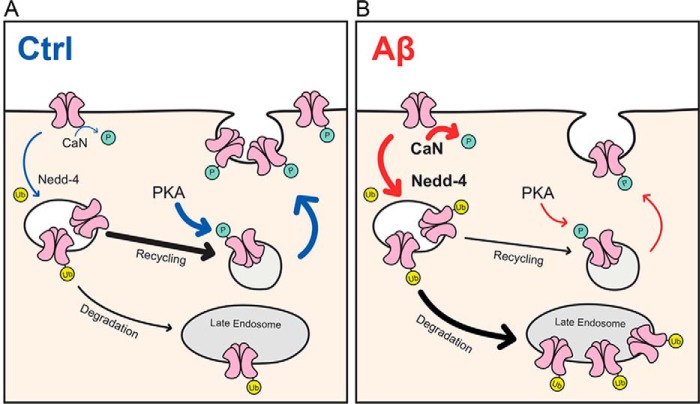
Figure 7.
Proposed model for the role of protein ubiquitination in mediating Aβ-induced down-regulation of surface AMPARs. A, under normal conditions, the majority of internalized AMPARs are recycled back to the plasma membrane, a process that is facilitated by PKA phosphorylation of the GluA1 subunit at Ser-845. The phosphorylation of GluA1 prevents the ubiquitination of AMPARs as a result of which receptor degradation is maintained at a low level for normal protein homeostasis. B, however, in the presence of high concentrations of soluble oligomeric Aβ, AMPARs are constantly endocytosed due to overactivation of the protein phosphatase calcineurin (CaN) and the E3 ubiquitin ligase Nedd4-1. This in turn leads to an increase in the level of GluA1 ubiquitination in part due to the down-regulation of Ser-845 phosphorylation. As a consequence, the recycling of AMPARs back to the plasma membrane (and therefore their expression on the cell surface) decreases, leading to synaptic depression. Ctrl, control; Ub, ubiquitin.