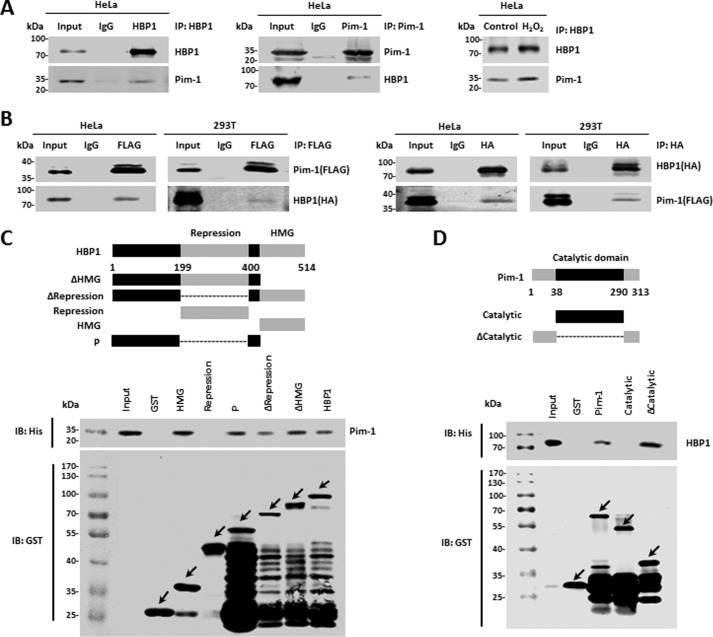
Figure 3.
Pim-1 interacts with HBP1 in vivo and in vitro. A and B, Pim-1 interacts with HBP1 in vivo, and H2O2 enhances the interaction. HeLa cells treated with or without H2O2 were lysed with IP lysis buffer and then subjected to immunoprecipitation with anti-HBP1 or anti-Pim-1 antibodies followed by Western blotting with anti-HBP1 or anti-Pim-1 antibodies (A). HeLa or HEK293T cells were cotransfected with FLAG-Pim-1 and HA-HBP1. An IP assay was carried out using anti-FLAG/HA antibody followed by Western blotting with anti-HA or anti-FLAG antibodies. The same samples were immunoblotted (IB) against FLAG or HA to determine immunoprecipitation efficiency (B). C and D, Pim-1 interacts with HBP1 in vitro. Shown is a schematic representation of N-terminal GST-tagged full-length HBP1 along with its various deletion mutants (C, top) and Pim-1 along with its various deletion mutants (D, top). P domain, the regions outside of the HMG box binding and the repression domains. GST pulldown assay was carried out to determine the domain of HBP1 essential for its interaction with Pim-1 (C, bottom) and the domains of Pim-1 essential for its interaction with HBP1 (D, bottom). GST pulldown efficiency was evaluated by Western blotting with anti-GST antibody. Arrows represent the proteins of GST-tagged HBP1, Pim-1, or their deletion mutants.