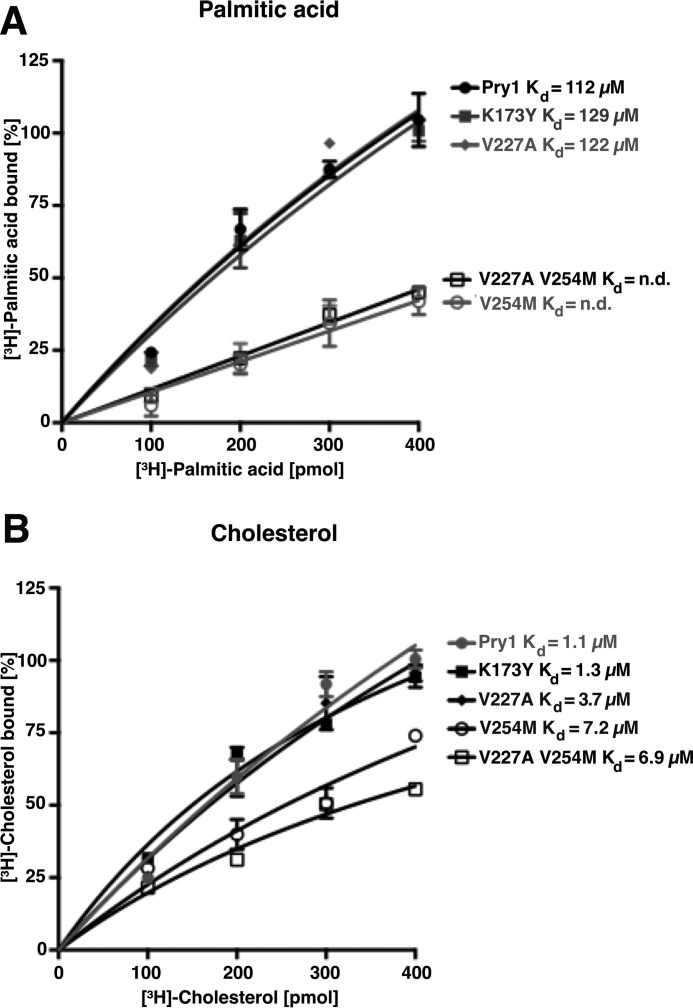
Figure 4.
Mutations within the fatty acid-binding pocket of Pry1 abrogate binding of fatty acids but not that of sterols. A, valine 254 is crucial for in vitro binding of palmitic acid by Pry1. The indicated residues in the fatty acid-binding pocket of Pry1 were mutated, and in vitro binding of [3H]-palmitic acid (100–400 pmol) to the wild-type (wt) and mutant versions of Pry1 was assessed. No Kd values can be determined for the V254M single and the V227A,V254M double mutant (n.d.). B, mutations in the fatty acid-binding pocket do not strongly affect sterol binding by Pry1. The indicated point-mutant versions of Pry1 (100 pmol) were incubated with [3H]-cholesterol (100–400 pmol) and ligand binding was quantified. Values represent means ± S.D. of three independent determinations.