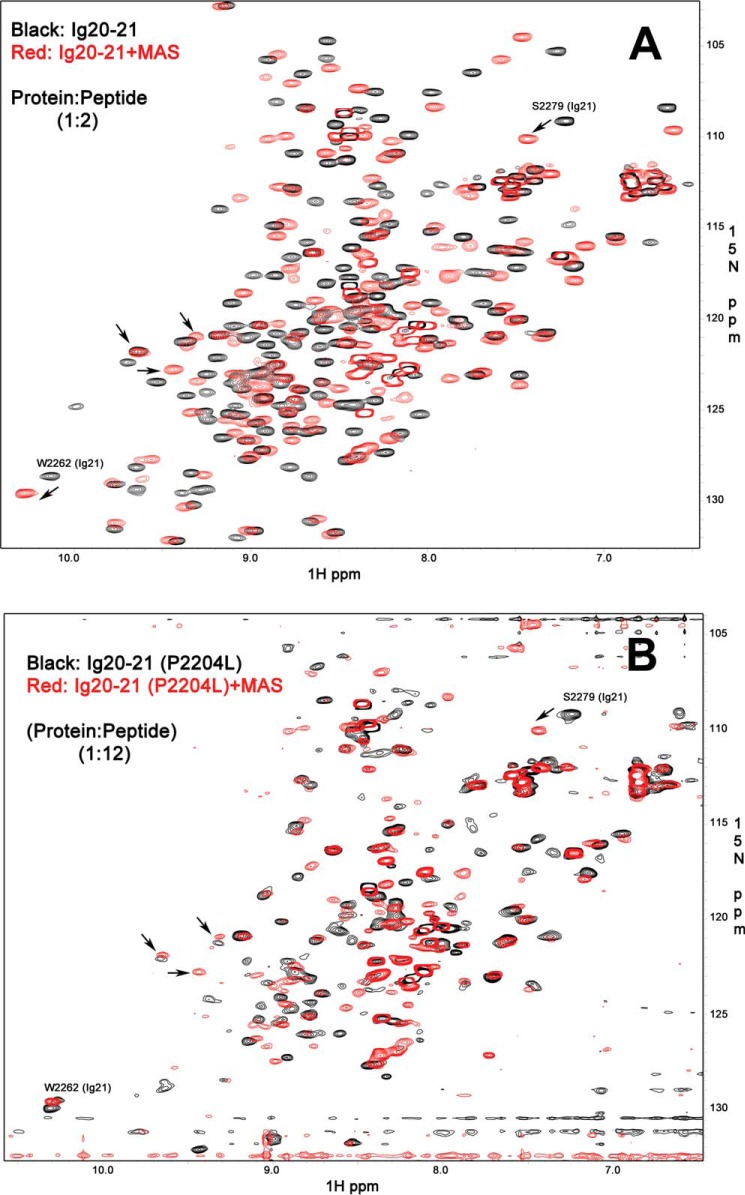
Figure 4.
Comparison of ligand binding to WT and mutant filamin. A, 15N HSQC of the WT Ig20–21 in the free form (0.2 mm WT protein, 8 scans, black) and with the bound MAS peptide (0.2 mm protein with 0.4 mm MAS peptide, 8 scans, red). B, 15N HSQC of the mutant P2204L Ig2021 in the free form (0.1 mm P2204L mutant, 64 scans, black) and with the bound MAS peptide (0.1 mm protein with 1.2 mm MAS peptide, 64 scans, red). Both proteins bind the MAS peptide as seen by the appearance of multiple new peaks, but the mutant protein has much broadened signals and does not recover the features of single conformational class upon ligand binding. Some peaks in >9 ppm range are marked by arrows to show that the protein has folded features but under dynamic intermediate exchange (molten globule). Such molten globule still binds to ligand as shown by characteristic shift of Ser-2279 in up-field, but the shift is less than that of WT (Fig. 4A), suggesting weaker binding.