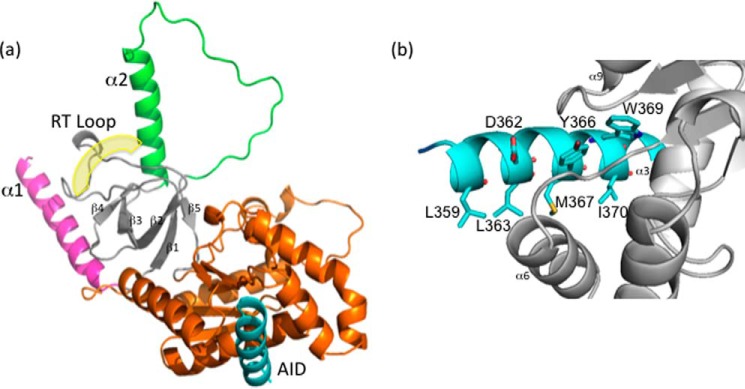
Figure 3.
X-ray crystal structure of DHPR β1a-SH3/GK complexed with AID peptide. a, cartoon representation of the DHPR β1a-SH3/GK complexed with AID. The split architecture of the SH3 domain is shown in gray with its labeled RT-loop highlighted. The RT-loop is sandwiched between the α1 (pink) and α2 helices (green), which are involved in the occlusion of the polyproline binding site. The GK domain is displayed in orange, and the DHPR I-II AID peptide binding ligand is in blue. The yellow shading denotes the putative polyproline binding site. b, close-up of the interaction between the AID peptide (blue) and β1a-SH3/GK (gray) highlighting contributing residues facilitating AID binding.