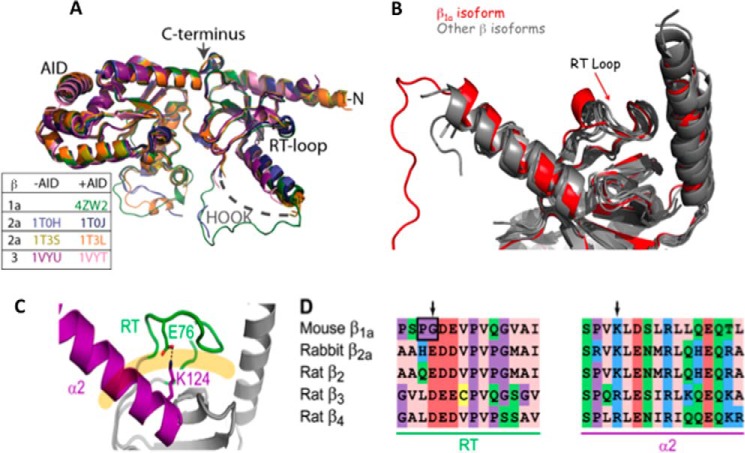
Figure 7.
Structural comparisons between β1a isoforms. A, backbone superposition of β1a with structures that have been crystallized with and without AID peptide. The color key corresponds to β isoforms. B, overlay of β X-ray crystal structures showing the SH3 domain, the α1 and α2 helices, and the RT-loop. The β1a structure is depicted in red. C, the X-ray crystal structure of the SH3 domain of rabbit β2a (PDB code 1t3l). The α2 helix and the RT-loop are highlighted in magenta and green, respectively, and interact through a salt bridge involving the side chains of Glu76 and Lys124 (shown). These structural elements occlude the polyproline binding site, which is displayed as a pale orange line. The sequence alignment of the α2 helix and the RT-loop is displayed for all β-subunit isoforms with the arrows denoting charged residues involved in a salt bridge that is absent in the β1a RT-loop.