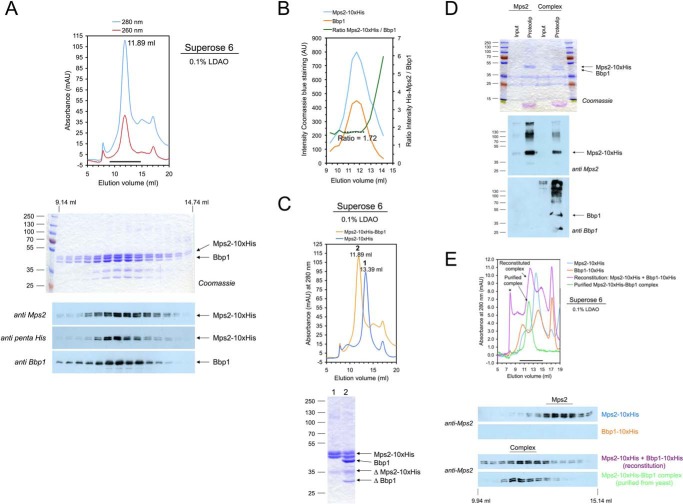
Figure 1.
Purification and reconstitution of the Mps2-Bbp1 membrane protein complex. A, purification of the Mps2-His10-Bbp1 protein complex from TKY384 yeast cells. Membrane proteins of galactose-induced TKY384 cells were solubilized with 1% LDAO, and Mps2-His10 was purified by IMAC and then analyzed by gel filtration on a Superose 6 column (upper panel) and subsequent SDS-PAGE and immunoblotting using anti-Mps2, anti-Bbp1, and anti-penta-His antibodies (lower panel). mAU, milliabsorbance units. B, the Mps2:Bbp1 ratio (as determined by quantifying the Coomassie Blue R250-stained gel shown in A with ImageJ; AU, absorbance units) was ∼1.7 and constant in the peak fractions of the gel filtration chromatography. C, comparison of Mps2 (blue line) and Mps2-Bbp1 (orange line) gel filtrations (upper panel). The peak fractions were analyzed by SDS-PAGE and Coomassie staining (lower panel). ΔMps2-His10 and ΔBbp1 are degradation products of Mps2-His10 and Bbp1, respectively. D, reconstitution of Mps2 and Mps2-Bbb1 complex into liposomes. Formation of proteoliposomes was analyzed by SDS-PAGE and subsequent Coomassie staining (upper panel) and immunoblotting (anti-Mps2/anti-Bbp1 antibodies; lower panel), respectively. Input, protein-lipid mixture diluted below critical micelle concentration (CMC) of the detergent and mixed with Nycodenz solution; Proteolip, floated proteoliposome fractions obtained after centrifugation. To visualize flotation of the formed proteoliposomes, the same volumes of inputs and floated proteoliposome fractions were analyzed. E, reconstitution of the Mps2-Bbp1 complex from purified Mps2-His10 and Bbp1-His10 proteins. Mps2-His10 and Bbp1-His10 were mixed in a molar ratio of ∼1:1, incubated for 15 min at room temperature, and then analyzed by gel filtration on a Superose 6 column (upper panel). In control experiments Mps2-His10, Bbp1-His10, and gel filtration-purified Mps2-His10-Bbp1 complex were treated in the same way. Proteins eluted from the Superose 6 column were analyzed by immunoblotting using an anti-Mps2 antibody (lower panel). The formed Mps2-His10-Bbp1-His10 complex eluted at ∼12.24 ml, and an additional “high molecular weight” Mps2-His10-Bbp1-His10 complex is marked with an asterisk. The reconstituted Mps2-His10-Bbp1-His10 complex could not be analyzed for the presence of Bbp1 because our Bbp1 antibody did not detect C-terminally tagged Bbp1. An anti-penta-His antibody did not allow discrimination of the similar-sized Mps2-His10 and Bbp1-His10 proteins.