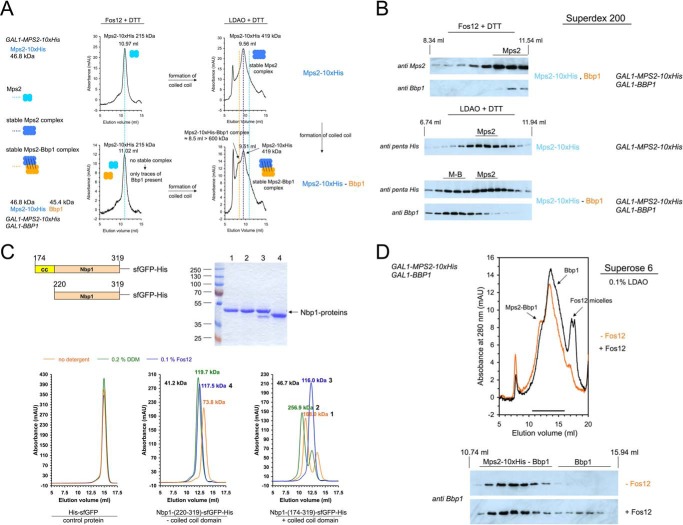
Figure 2.
The stability of the Mps2-Bbp1 complex is based on coiled-coil domain interactions. A, purification of the Mps2-Bbp1 complex failed using Fos12 as detergent. Mps2-His10 (upper panel) and Mps2-His10-Bbp1 (lower panel) were purified from galactose-induced yeast TKY376 and TKY377, cells respectively. The copy number of the BBP1 gene in TKY377 cells is lower than in TKY384 cells so that in this case not all purified Mps2 protein was in the Mps2-Bbp1 complex (compare Fig. 1 and supplemental Fig. S2A). Mps2 and Mps2-Bbp1 each were purified using the detergents Fos12 (left panel) and LDAO (right panel) and analyzed by gel filtration on a Superdex 200 column. Complexes are shown in a schematic way, because the exact number of monomers is not known. mAU, milliabsorbance units. B, immunoblot analysis of Mps2-Bbp1 purifications in the presence of Fos12 and LDAO, respectively. The fractions from the gel filtration experiments in A were analyzed by immunoblotting with anti-Mps2, anti-Bbp1, and anti-penta-His antibodies, respectively. The Mps2-Bbp1-complex (M-B) was only detectable when LDAO was used as detergent. The elution volume of Mps2 clearly depended on the used detergent und was significantly decreased in the Mps2-Bbp1 complex. C, the apparent molecular weight of the C-terminal Nbp1 domain was changed in the presence of Fos12 detergent. sfGFP-His6 (control protein), Nbp1-(174–319)-sfGFP-His6 (having a strong N-terminal coiled-coil (CC) domain from amino acids 174–219; 46.7 kDa), and Nbp1-(220–319)-sfGFP-His6 (41.2 kDa) were purified from E. coli, adjusted to 10 mm DTT, and incubated for 5 min at 4 °C with no detergent, 0.2% DDM, or 0.1% Fos12. Gel filtration on a Superdex 200 column using different buffer conditions (no detergent versus 0.2% DDM versus 0.1% Fos12 in 50 mm Tris/HCl, pH 8.0, 300 mm NaCl, 10% glycerol buffer) was followed by absorbance at 490 nm. The peak fractions (1–4) of the gel filtration runs were analyzed by SDS-PAGE to verify the correct sizes of monomeric proteins. D, the Mps2-Bbp1 complex purified using LDAO as detergent was disrupted by the addition of Fos12. Mps2-His10-Bbp1 was purified from galactose-induced yeast TKY377 cells using LDAO as detergent. Then, to one aliquot of the IMAC eluate, 1% Fos12 was added, and after 5 min of incubation at room temperature the reaction mixtures were analyzed by gel filtration on a Superose 6 column (with 0.1% LDAO in the running buffer). The eluted fractions were analyzed by immunoblotting with anti-Bbp1 antibodies.