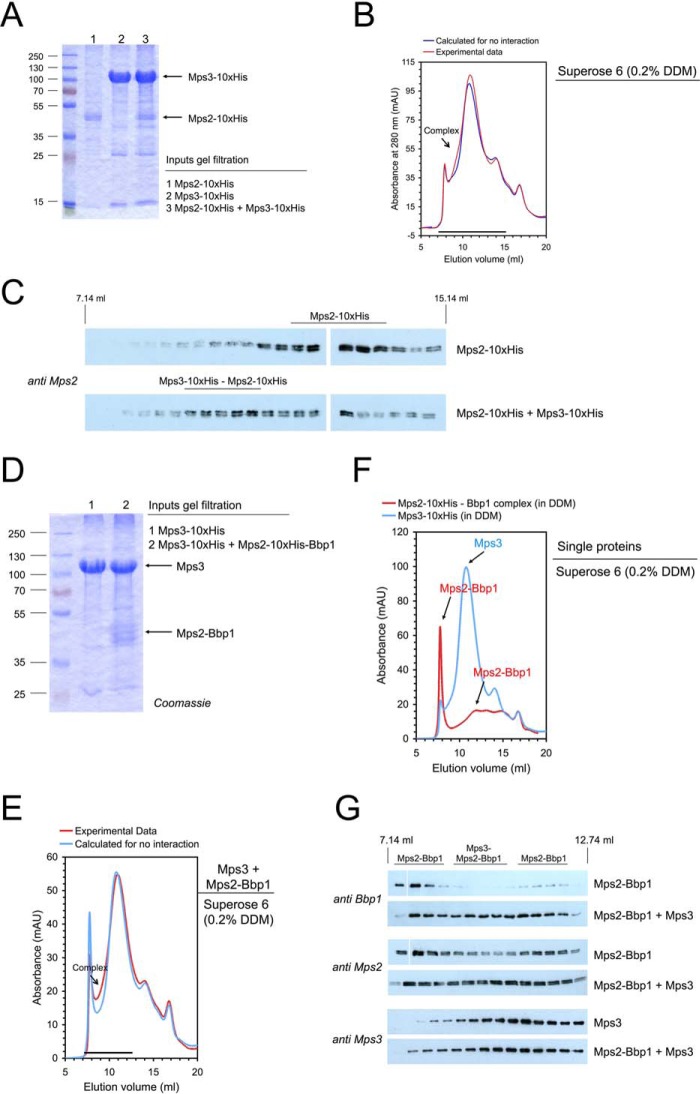
Figure 3.
Interaction of the SUN-domain protein Mps3 with the Mps2-Bbp1 complex. A–C, reconstitution of a Mps3-Mps2 complex. A, Mps3-His10 and Mps2-His10 were separately purified by solubilization with DDM and subsequent IMAC. Mps3 (in molar excess) was mixed with Mps2 and analyzed by SDS-PAGE and Coomassie staining. B, gel filtration of Mps2-His10, Mps3-His10, and the Mps3-His10-Mps2-His10 mixture was performed on a Superose 6 column (red line, experimental data; blue line, calculated data if Mps2-His10 and Mps3-His10 do not interact). mAU, milliabsorbance units. Complex formation is visible as a shoulder in the chromatogram. C, proteins eluted from the Superose 6 column were analyzed by immunoblotting with anti-Mps2 antibodies. The formation of the Mps3-Mps2 complex was visible as an increase of the Mps2 blot signal intensity at an elution volume of ∼9 to 11 ml. D–G, reconstitution of the Mps3-Mps2-Bbp1 complex. Mps3-His10 and the Mps2-His10-Bbp1 complex were separately purified by solubilization with DDM and subsequent IMAC. Mps3 (in molar excess) was mixed with Mps2-Bbp1 (inputs were analyzed by SDS-PAGE and Coomassie staining: 1, Mps3-His10; 2, Mps3 + Mps2-His10-Bbp1) (D) and analyzed by gel filtration on a Superose 6 column (complex formation is visible as a shoulder in the chromatogram) (E). F, in control experiments the Single proteins Mps3-His10 and the Mps2-His10-Bbp1 complex were treated in the same way. Elution from the gel filtration column was followed by absorbance at 280 nm (F) and immunoblotting with anti-Bbp1, anti-Mps2 and anti-Mps3 antibodies, respectively (G).