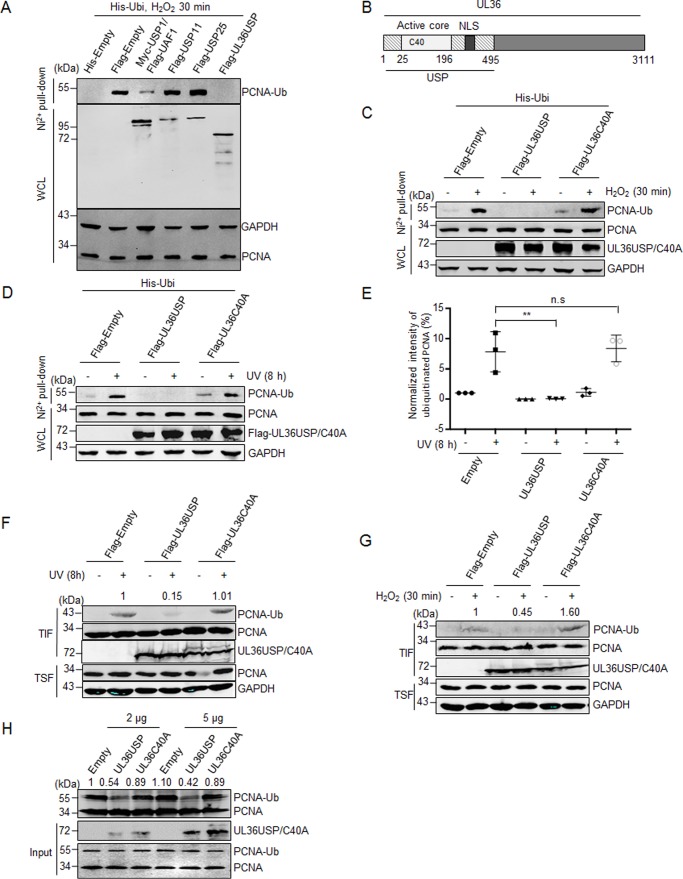
Figure 1.
UL36USP deubiquitinates PCNA in vivo and in vitro. A, UL36USP reduces PCNA ubiquitination more efficiently than USP1/UAF1. HEK293T cells were cotransfected with His-ubiquitin and the indicated deubiquitinase plasmids, at 48 h after transfection, cells were treated with 100 μm H2O2 for 30 min and lysed. Cell lysates were subjected to His pulldown analysis, and the ubiquitinated PCNA was detected by Western blotting using anti-PCNA antibody. B, UL36USP is the N-terminal region of the HSV large tegument protein UL36. The cysteine at position 40 is the key amino acid of deubiquitinating activity. C–G, wild-type UL36USP, but not the C40A mutant, deubiquitinates PCNA in response to DNA damage induced by H2O2 or UV treatment. HEK293T cells were transfected with UL36USP or mutant UL36C40A that were defective in deubiquitination. At 40 h after transfection, cells were stimulated with or without H2O2 (100 μm, C) or UV (40 J/m2, D) and subjected to a His pulldown assay as described above. E, quantification of ubiquitinated PCNA normalized to the empty vector expressing group. Three independent His pulldown experiments were performed at the same conditions of D, the normalized intensities of PCNA ubiquitination was shown in scatter plots with standard deviation to represent error. F and G, HEK293T cells were transfected with UL36USP or UL36C40A for 40 h, and then treated with or without UV (F) or H2O2 (G). Cells were harvested, incubated with Triton X-100 buffer, and fractionated into chromatin-containing insoluble (TIF) and soluble (TSF) fractions. PCNA were concentrated in the insoluble fraction and probed by anti-PCNA antibody. The intensity of ubiquitinated PCNA was normalized to the total quantity of PCNA in TIF. H, in vitro deubiquitination assay shows UL36USP but not C40A deubiquitinates PCNA. HEK293T cells were transfected with His-ubiquitin and HA-PCNA plasmids. At 48 h after transfection, UV was used to promote PCNA ubiquitination. Ubiquitinated PCNA was precipitated by using Ni2+ beads and incubated with purified UL36USP or UL36C40A recombinant protein, as indicated, at 30 °C overnight, and then detected by Western blotting with antibody targeting PCNA. The intensity of ubiquitinated PCNA was normalized to the total quantity of PCNA calculated by adding ubiquitinated and unmodified PCNA. UL36USP and UL36C40A proteins were purified from E. coli. All the experiments described have been repeated more than 3 times. WCL, whole cell lysates; **, p value < 0.01; n.s., not significant.