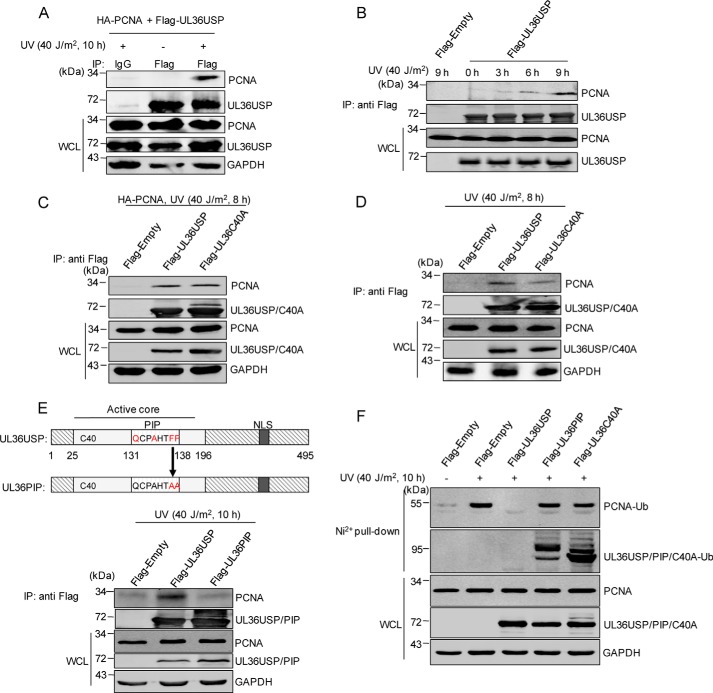
Figure 2.
UL36USP and UL36C40A interact with PCNA in response to DNA damage. A and B, UV treatment enhances the interaction between PCNA and UL36USP. HEK293T cells transfected with FLAG-UL36USP and HA-PCNA plasmids were treated with or without UV for the indicated time. After stimulation, a co-IP assay was performed to assess the interaction between PCNA with UL36USP or -C40A using the indicated antibodies. C, PCNA interacts with both UL36USP and UL36C40A. HEK293T cells transfected with HA-PCNA, FLAG-Empty, FLAG-UL36USP, or UL36C40A plasmids were treated with 40 J/m2 UV. At 8 h post-stimulation, cells were harvested and subjected to co-IP experiment. D, endogenous PCNA interacts with UL36USP and UL36C40A. HEK293T cells were transfected with FLAG-empty, FLAG-UL36USP, or UL36C40A plasmids and harvested as described in B. E, a schematic diagram of the UL36USP PIP domain and the UL36PIP mutation construct. The conserved amino acids of PIP domain are indicated by red, and the contiguous phenylalanines were mutated to alanines. The interaction between PCNA and UL36USP or UL36PIP was assessed by co-IP analysis using anti-FLAG® M2 magnetic beads, followed by Western blotting with anti-PCNA antibody. F, UL36PIP does not inhibit PCNA ubiquitination. HEK293T cells transfected with the indicated plasmids were subjected to 40 J/m2 UV stimuli for 10 h. And then a His pulldown assay was performed to examine ubiquitinated PCNA.