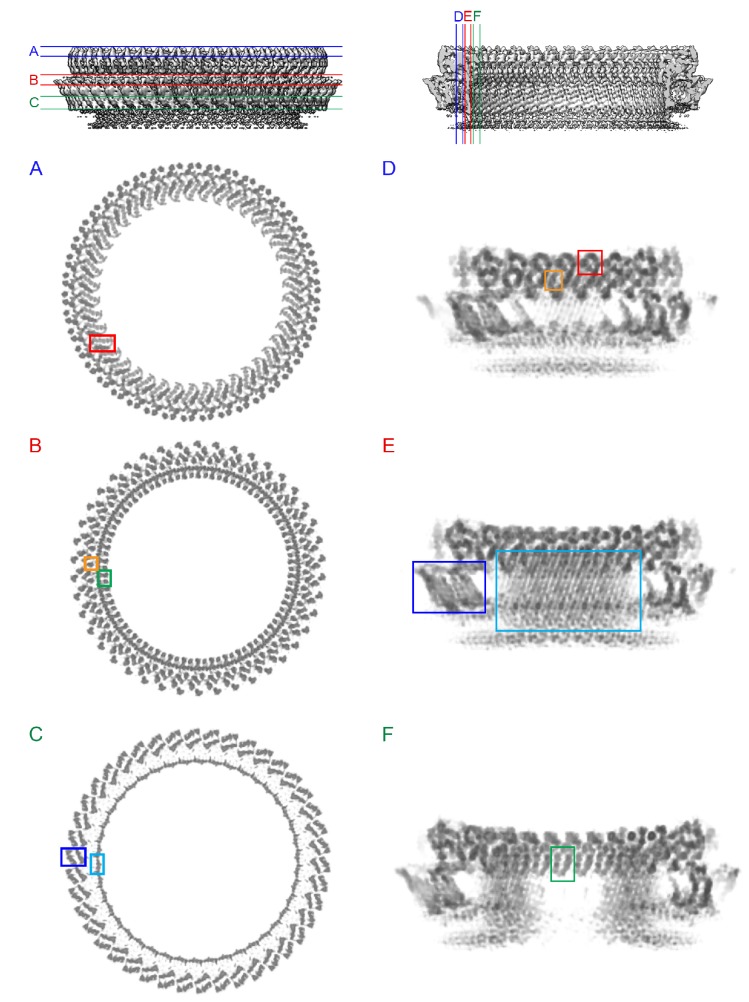
Figure 4. Intermolecular interactions in the 4.5 Å cryoEM map of the PLY pore complex.
Slices parallel (A–C) and perpendicular (D–F) to the membrane reveal interactions of secondary structure elements between adjacent monomers in the pore complex. Section planes are indicated on top. Note that the scale of sections D to F is 50% larger to show molecular detail more clearly. The β5-α4-β6 region in domain D1 and the long membrane-parallel helix α5 (red box in A and D) alternate around the top of the pore complex. On the outside, the 168-strand β-barrel is flanked by helix α3a (orange box in B and D) and by the α-barrel of the helix-turn-helix motifs (green box in B and F) on the inside. The 4-strand β-sheets of domain D4 (blue box in C and E) are offset against D4 of the next monomer, forming a ring of 8-strand β-sheets. The 85 Å-long β-strands forming the 168-strand β-barrel are clearly resolved (cyan box in C and E).