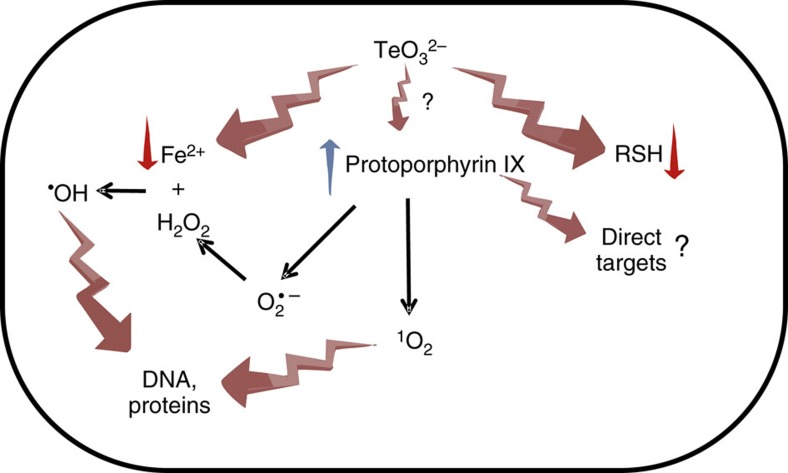
Figure 6. Schematic model for tellurite and proto IX toxicity in E. coli.
Once inside the cell, tellurite depletes the pool of reduced thiols (RSHs) and targets iron–sulfur clusters. As a response, E. coli decreases the pool of intracellular iron, substrate for the enzyme ferrochetalase together with proto IX, causing its accumulation. Alternatively, tellurite could directly inactivate enzymes of the last two steps of heme biosynthesis (?), resulting in protoporphyrin IX accumulation. Protoporphyrin IX directly targets unidentified cellular targets (?) and also acts as a substrate for the production of superoxide (O2·−) and/or singlet oxygen (1O2) by electron or energy transfer, respectively. The O2·− is dismutated to hydrogen peroxide (H2O2), which reacts with the free Fe2+ (Fenton reaction) derived from the direct action of tellurite, generating the highly toxic hydroxyl radical (·OH). As E. coli does not encode enzymes for the detoxification of ·OH or 1O2 and the defensive RSHs are depleted, these ROS, together with protoporphyrin IX, target intracellular macromolecules, causing cell death. TeO32−: tellurite.