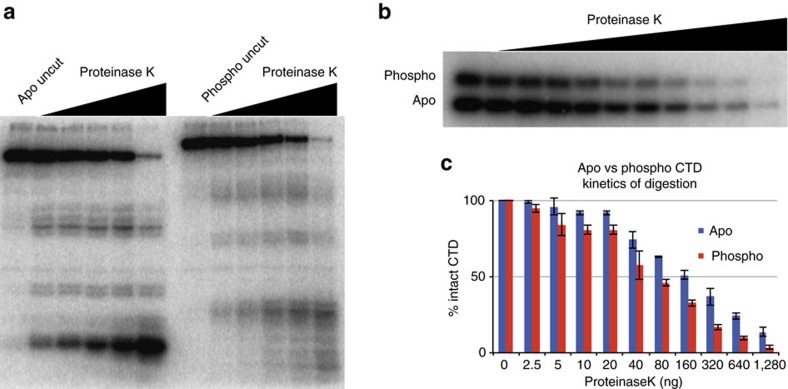
Figure 5. Local structural heterogeneity is maintained in the phospho CTD but the phospho CTD is more accessible to protein interaction.
(a) Limited proteolysis of the phospho CTD shows an altered pattern of proteolysis. The hypersensitivity of the distal site is reduced, and the pattern of proteolysis is more evenly distributed among the sensitive sites. The proximal sensitive region, and central protected region are preserved after phosphorylation, with proteolytic fragments shifted relative to the apo fragments due to phosphorylation across the CTD. (b) Representative limited proteolysis of a mixture of apo and phospho CTD to compare protease accessibility. (c) Average of three replicates of the apo (blue bars) and phospho (red bars) mixing experiments quantified as the percentage of intact CTD remaining at each protease concentration. 100–50% intact CTD is the single hit kinetics range of the experiment42. Error bars depict s.e.m.