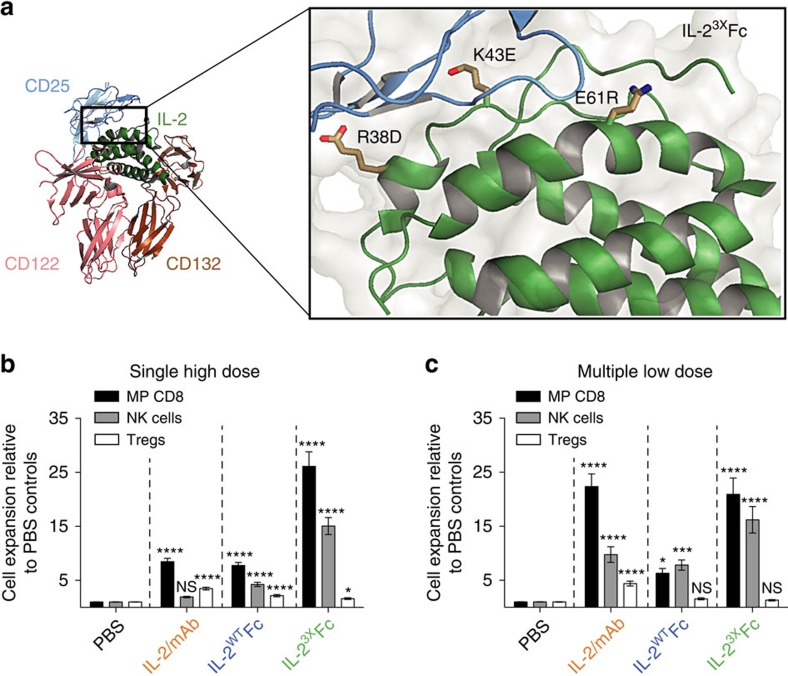
Figure 1. Abolition of CD25 binding results in potent and selective expansion of cytotoxic lymphocyte subsets.
(a) Selected charge-reversal mutations were introduced into human IL-2 based on inspection of the IL-2/IL-2R co-crystal structure32 (PDB: 2B51), with the aim of disrupting CD25 binding. Highlighted are the mutations introduced to generate the IL-23XFc triple mutant. R38D and E61R were selected after binding kinetics and cell-based assays (Supplementary Figs 1 and 2), while K43E was incorporated after screening of in vivo activity (Supplementary Fig. 4A–G). (b,c) Lymphocyte expansion profiles in the spleens of C57BL/6 mice receiving IL-2/mAb, IL-2WTFc or IL-23XFc treatment. (b) Fold expansion in the total numbers of memory-phenotype CD8+ T-cells (CD8+ CD44high CD122high), NK cells (CD3− NK1.1+ CD122high) and Tregs (CD4+ FoxP3+ CD25+) after a single IL-2/mAb (3 μg IL-2+15 μg mAb) or IL-2-Fc (16.8 μg) i.p. injection was determined by flow cytometry on day 5. Shown is pooled data from seven independent experiments, each normalized to the average subset numbers of two to three PBS-treated mice (PBS, n=16; treatment groups, n=4–8). (c) Fold expansion in the total numbers of MP CD8, NK cells and Tregs after five IL-2/mAb (1 μg IL-2+5 μg mAb) or IL-2-Fc (5.6 μg) i.p. injections (days 0–4) was determined by flow cytometry on day 5. Shown is pooled data from five independent experiments, each normalized to the average subset numbers of two to three PBS-treated mice (PBS, n=18; treatment groups, n=8). Data are displayed as mean±s.e.m. Asterisks indicate significant differences relative to PBS controls (*P<0.05, ***P<0.001, ****P<0.0001) as determined by one-way analysis of variance with Bonferroni post hoc test for multiple comparisons.