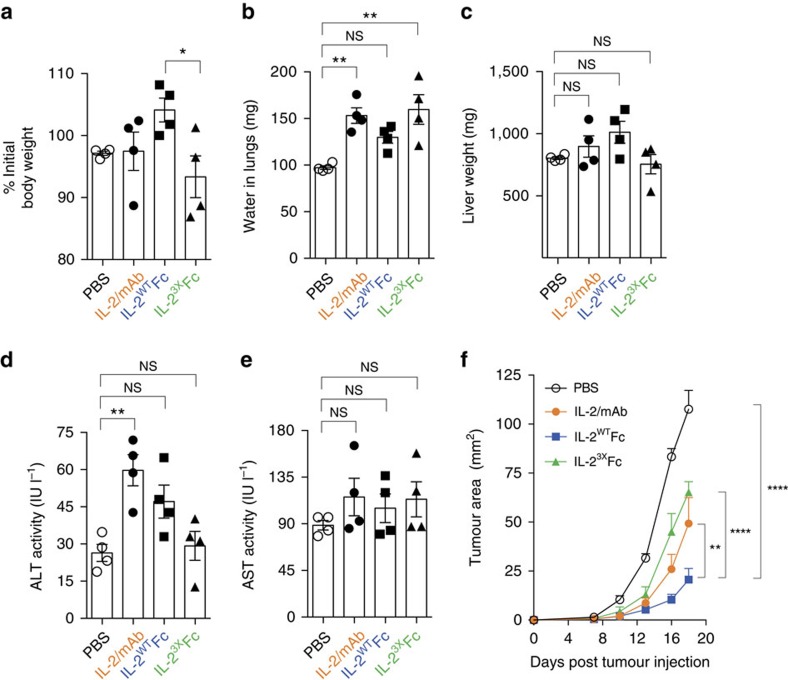
Figure 2. Toxicity profile and antitumour activity of IL-2-Fc variants.
(a–e) Symptoms of experimental VLS were assessed in mice receiving five consecutive doses (days 0–4) of IL-2/mAb (1 μg IL-2+5 μg mAb per dose), IL-2WTFc (5.6 μg per dose), IL-23XFc (5.6 μg per dose) or PBS control. Lungs, livers and blood were collected on day 6 for analysis (n=4 mice per group). (a) Body weight on day 6, represented as percentage of initial body weight on day 0. (b) Pulmonary oedema was assessed by measurement of lung water content, with significantly higher accumulation of fluid observed in the IL-2/mAb (P=0.0042) and IL-23XFc (P=0.0018) groups, compared to PBS controls. (c–e) Assessment of liver toxicity as measured by total liver weight (c) and serum levels of liver enzymes alanine aminotransferase (ALT, d) and aspartate aminotransferase (AST, e). (f) Tumour growth after subcutaneous inoculation of B16F10 melanoma cells into the flanks of mice treated with five consecutive doses of IL-2/mAb (1 μg IL-2+5 μg mAb per dose), IL-2WTFc (5.6 μg per dose), IL-23XFc (5.6 μg per dose) or PBS control on days 1–5 (n=6). Data are displayed as mean±s.e.m. Asterisks indicate significant differences between specified groups (*P<0.05, **P<0.01, ****P<0.0001) as determined by one-way analysis of variance (ANOVA) (a–e) or two-way ANOVA (f) with Bonferroni post hoc test for multiple comparisons.