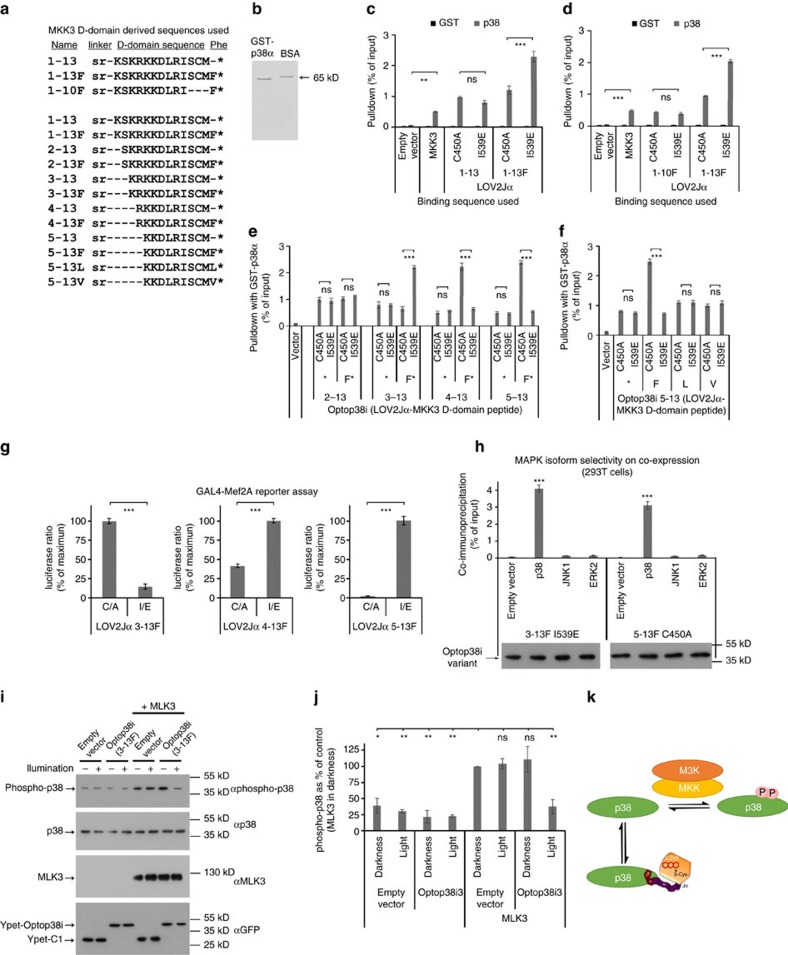
Figure 10. Optop38i design and functional validation.
(a) Alignment of putative p38MAPK inhibitor sequences is shown. (b) Representative coomassie-stained gel of immobilized recombinant p38 calibrated against 0.25 μg BSA (65 kDa). (c) Luciferase-fused Optop38i 1–13 and 1–13F, dsm (C450A) and lsm (I539E), were evaluated by pulldown assay. All variants were efficiently pulled down by GST-p38, comparable to positive control Luc-MKK3b. Only addition of C-terminal phenylalanine resulted in significant change in pulldown between dsm and lsm (n=6 or 3 for 1–13). (d) Pulldown of luciferase-fused Optop38i 1–10F and 1–13F (dsm and lsm) was compared. Removal of C-terminal residues 11–13 eliminated lsm/dsm differences in pulldown (n=3). (e) N-terminal iterative trimming of inhibitor sequence with or without C-terminal phenylalanine was carried out to optimize lsm/dsm ratio. Optop38i 3–13F showed a larger lsm/dsm pulldown ratio than earlier variants. Shorter sequences 4–13F and 5–13F showed reversed dsm/lsm selectivity with the greatest pulldown ratio detect using 5–13F; removal of C-terminal phenylalanine eliminated dsm/lsm differences (n=3). (f) GST-p38 pulldowns with Optop38i 5–13, 5–13F, 1–13L or 5–13V showed that only Optop38i 5–13F exhibited LOV2Jα-state dependent binding (n=3). (g) Optop38i effects on p38-dependent transcription. Pairwise comparison of YFP-Optop38i lsm and dsm cotransfected 24 h with GAL4-Mef2A, p38 and constitutively active MKK3, produced lower reporter activity with Optop38i-state mutants showing stronger pulldown in Fig. 10e (3–13F.lsm, 4–13F.dsm, 5–13F.dsm). Statistical significance was evaluated by two-tailed t-test (n=3). (h) Optop38i 3–13F.lsm or 5–13F.dsm co-immunoprecipitated with p38MAPK but not JNK, ERK2 or vector. The representative immunoblots confirm equal Ypet-Optop38i expression (n=3). (i,j) 283T cells were transfected with MLK3 and Optop38i3 (wild-type 3–13F) or empty vectors for ∼20 h. Pulsed blue light was applied for 1 h. (i) Immunoblot of lysates show illumination of Optop38i3-expressing cutures inhibited MLK3-evoked phosphorylation of endogenous p38. Representative blots with loading control blots are shown. (j) Quantified replicates (n=3) and significance of differences are shown. (k) Scheme depicting inhibition of MLK3-evoked p38 phosphorylation by illuminated Optop38i3 as in figures. (i,j) Mean±s.e.m. is indicated, ns not significant, *P<0.05, **P<0.01, ***P<0.001. Statistical analysis was carried out by one- or two-way ANOVA and Bonferroni post-test where not otherwise stated (Supplementary Data 1).