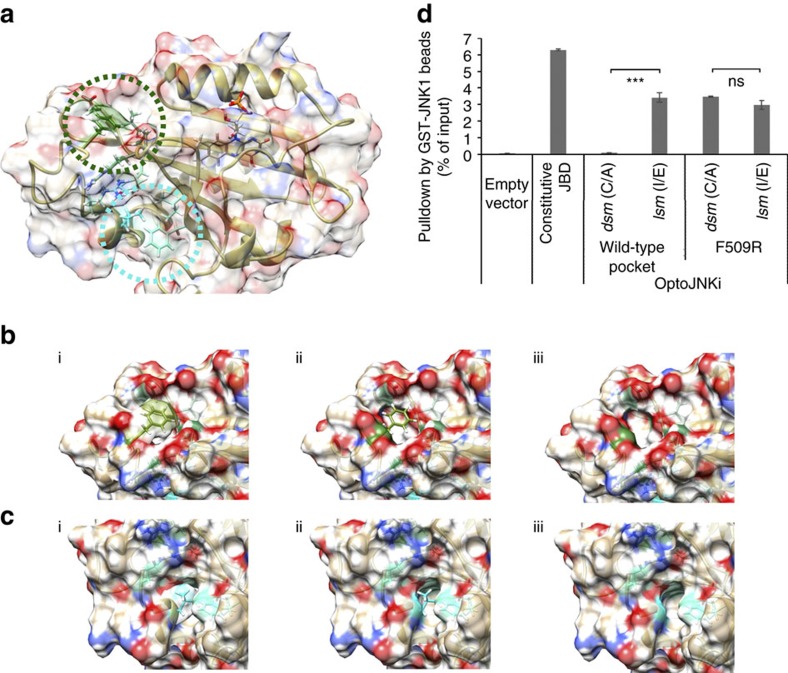
Figure 9. Molecular dynamics simulation of OptoJNKi suggests a potential photoregulation mechanism by hydrophobic tether capture in a new pocket.
(a) Representation of the OptoJNKi structure deduced from time-averaged atom positions from a molecular dynamics simulation (see ‘Methods' section). The Cα chain is shown in tan. The structure is overlaid with a semi-transparent molecular surface. The atoms of F559 (the C terminus of the protein) and L546, together with interacting neighbouring residues, are highlighted green and cyan (carbon), respectively. Regions surrounding F559 and L546 are encircled. (b) The molecular packing of the terminal phenylalanine residue into a distal hydrophobic pocket in the OptoJNKi structure, as deduced by molecular dynamics simulation, is shown. Atoms shown are coloured tan (carbon), white (hydrogen), blue (nitrogen) and red (oxygen). F559 atoms are highlighted green (carbon), as are its interacting neighbouring residues, P420, R421, D505, V506 and F509. The overlaid semi-transparent molecular surface emphasizes (i) the hydrophobic pocket of the F559, (ii) the partial ‘caging' of F559 depicted in stick format and (iii) the depth of the hydrophobic pocket created by F559 (which is itself not shown in biii to assist in visualization). (c) The corresponding molecular packing of the L546 residue into the Jα-proximal hydrophobic pocket in the optoJNKi structure as in b is shown. L546 atoms are highlighted cyan (carbon), as are its interacting neighbouring residues R549, Y508, I417 and F429. The overlaid semi-transparent molecular surface here emphasizes the hydrophobic pocket, full ‘caging' depth of the hydrophobic pocket created by L546 in (i)–(iii) as for F559 in Fig. 9b. (d) GST-JNK1 pulldown was performed as in Fig. 1 using the OptoJNKi (dsm and lsm) with a wide-type LOV2 pocket, the OptoJNKi (dsm and lsm) with LOV2 pocket mutant F509R, and the constitutive JBD (JIP1-277) as positive control. F509R mutants interacted with JNK1 similarly as wild-type OptoJNKi.lsm (I539E), failing to show any difference between lit- and dark-state mutants (n=3). Mean±s.e.m. is indicated, ns not significant, ***P<0.001. Analysis was carried out using by one-way ANOVA/Bonferroni post-test (Supplementary Data 1).