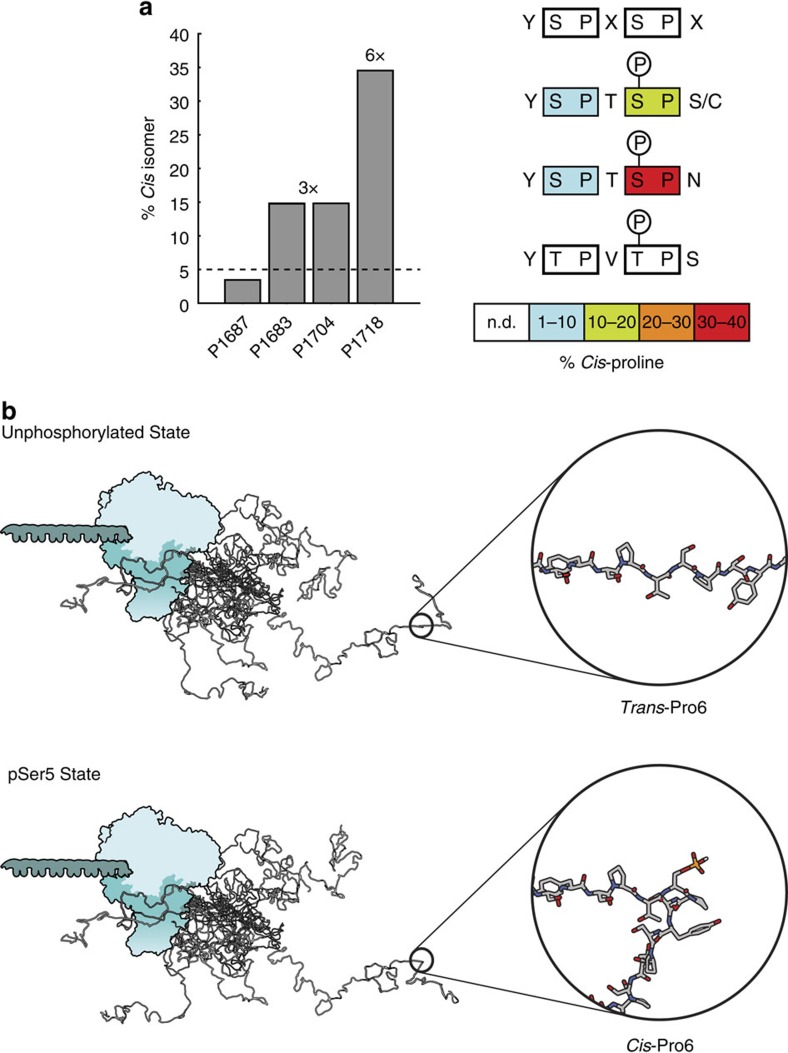
Figure 6. The impact of serine 5 phosphorylation on the structure of the Dm CTD.
(a) Percentage of cis-proline for several proline residues determined from peak intensities in 2D NMR correlation spectra of hyper-pSer5 CTD2′, where the dotted line denotes the average percentage of cis-proline in the unphosphorylated state (left). This is depicted schematically for various heptad sequences in CTD2′ (right). (b) Model for the effect of Ser5 phosphorylation on the structure of the CTD. In the unphosphorylated state, the CTD exists in an ensemble of conformational states that favour prolines in the trans conformation (top). Hyper-pSer5 incorporation causes the CTD heptad repeats bearing the sequence motifs highlighted in a to undergo dramatic structural rearrangement driven by pSer5-dependent proline isomerization.