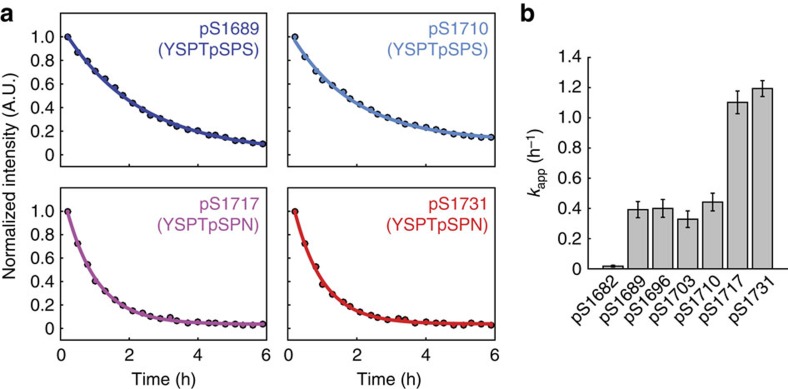
Figure 7. Structural switches in hyper-pSer5 CTD2′ modulate the apparent Ssu72 activity.
(a) Representative kinetic traces of Ssu72 dephosphorylation of pSer5 in CTD2′ monitored by RT-NMR. (b) Apparent rate constants for pSer5 dephosphorylation reveal heptad-specific Ssu72 activities. The highest apparent Ssu72 activities are observed for pSer5 residues within heptads containing Asn7. Error bars represent the errors from non-linear least squares fitting. All fitting procedures are described in detail in Methods section.