Figure 6. Conservation of ubiquitylation‐dependent oligomerization in human Nedd4 and implications in targeting of FGFR1.
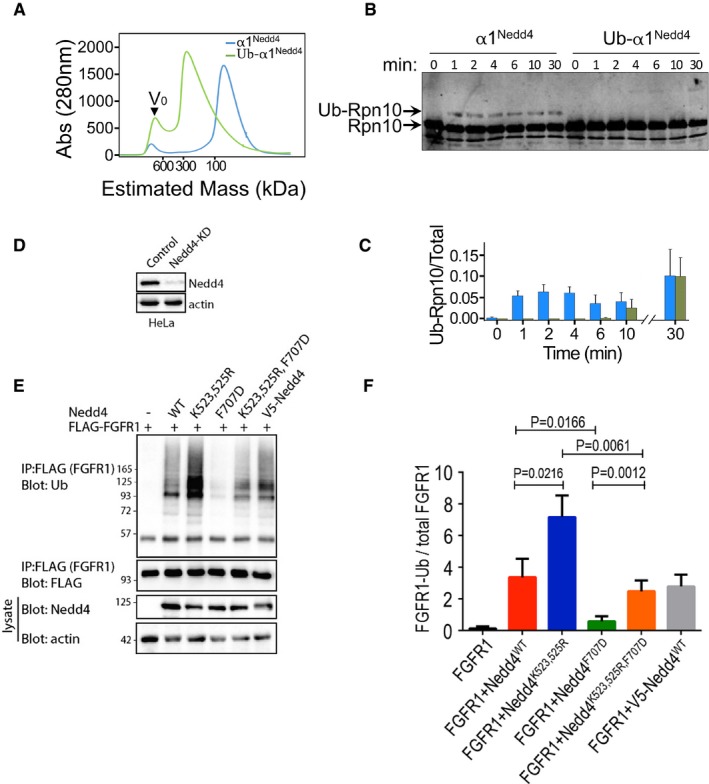
- Elution profile from size‐exclusion chromatography of apo HECT (α1Nedd4) or Ub‐fused HECT (α1Nedd4‐Ub) of Nedd4. Proteins were loaded on a Superdex 200 16/60 column, and elution was monitored by A280 measurements.
- In vitro ubiquitylation of Rpn10 by apo HECT (α1Nedd4) or Ub‐fused HECT (α1Nedd4‐Ub) of Nedd4. Reaction products were resolved by SDS–PAGE and blotted against a primary rabbit α‐Rpn10 antibody, followed by secondary IR labelled mouse α‐rabbit. Odyssey infrared imaging system was used for IR detection.
- Quantified ubiquitylated/total Rpn10 ratio (mean values and standard deviation bars from three replicates).
- Documentation of Nedd4 knockdown by shRNA in HeLa cells.
- Representative immunoblot of FGFR1 ubiquitylation (in the presence of serum) upon transfection of the indicated wild‐type (WT) and mutant human Nedd4 constructs. V5‐Nedd4 was used as an additional control.
- Quantified (mean ± SEM) ubiquitylated/total FGFR1 ratio from three separate experiments. P‐values are from Student's t‐test.
