Figure EV6. Specificity of ATG8 sensors.
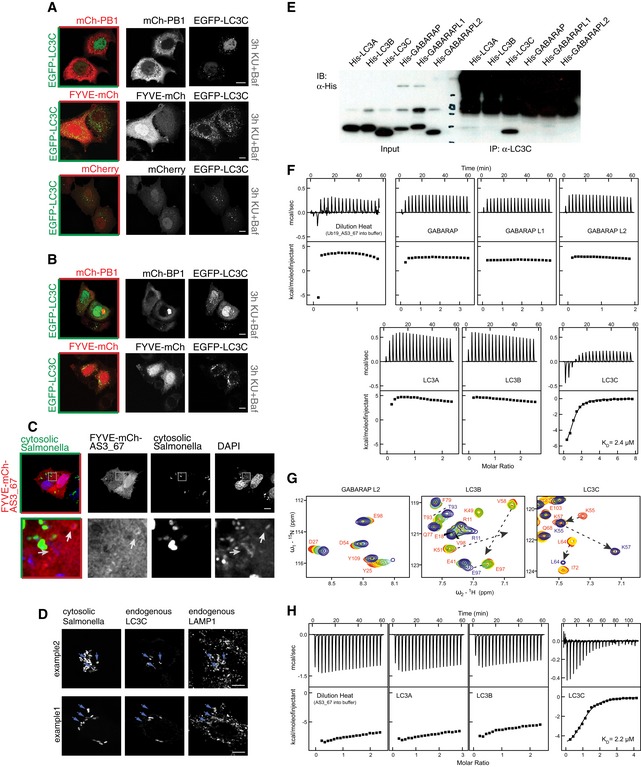
- A, B
-
CHigh‐performance sensor FYVE‐mCh‐AS3_67 was transiently expressed in Salmonella‐infected HeLa cells to monitor xenophagy events. Even though the sensor was recruited to cytosolic Salmonella to certain extent, its performance fell short of sensor mCh‐PB1‐AS3_67. Scale bar: 10 μm.
-
DA subpopulation of cytosolic Salmonella is coated with endogenous LC3C and engulfed by lysosomes in HeLa cells (marker: endogenous LAMP1). Arrows indicate examples of Salmonella located within lysosomes. Scale bar: 10 μm.
-
ELC3C‐specific antibody (Stadel et al, 2015) was incubated with purified, recombinant HIS6 fusion proteins of mATG8s and subsequently subjected to immunoprecipitation (IP) experiments with protein A–Sepharose. The specificity of the antibody for LC3C was confirmed. Unspecific signal in the high molecular weight area of the IP samples is caused by the LC3C antibody.
-
FITC experiments showed significant preference of Ub19‐AS_67 fusion construct to LC3C. Titrations of all other human ATG8 proteins with Ub19‐AS_67 fusion construct revealed weak interactions and do not differ significantly from Ub19‐AS_67 dilution heat. In contrast, titration of LC3C with Ub19‐AS_67 fusion construct shows a relatively strong interaction (K D 2.4 μM, ΔH −7.2 kcal/mol, and ΔS +1.4 cal/mol/K) comparable to that for p62 and NBR1 LIR interactions to LC3B (Rozenknop et al, 2011). Top panels display the raw heat per injection, and the lower panels display the integrated heat per titration over molar ratio (protein: Ub19‐AS_67).
-
GNMR titration of Ub19‐AS_67 fusion construct to 15N‐labeled GABARAPL2, LC3B, and LC3C proteins shows its specificity for LC3C. NMR titration of Ub19‐AS_67 fusion construct to 15N‐labeled GABARAPL2 (left plot) and LC3B (middle plot) indicates weak interactions with an intermediate exchange mode, while that for LC3C shows a stronger interaction in slow exchange mode. Representative sections of 1H‐15N HSQC spectra for 15N‐labeled proteins upon titration with Ub19‐AS_67 fusion construct are shown in overlay. All plots show “fingerprint regions” of the proteins spectra (around HN resonance of K51 in LC3B, K48 in GABARAPL2, and K57 in LC3C). Molar ratios of protein: Ub19‐AS_67 are rainbow‐color‐coded (1:0, 1:0.25, 1:0.5, 1:1, 1:2; red to blue) for each titration step. Changes in positions of resonances due to interaction with AS_67 are highlighted with dashed arrows.
-
HITC experiments showed significant preference of AS_67 peptide to LC3C. Similar to Ub19‐AS_67 fusion construct, de‐tagged AS_67 peptide did not show significant interaction with LC3A and LC3B proteins. Corresponding titration profiles are very similar to dilution heat of the peptides. In contrast, AS_67 peptide interacts with LC3C protein, and ITC analysis results are similar to Ub19‐AS_67 parameters of interaction (K D 2.2 μM, ΔH −5.4 kcal/mol, and ΔS +7.8 cal/mol/K). Top panels display the raw heat per injection, and the lower panels display the integrated heat per titration over molar ratio (protein: AS_67).
