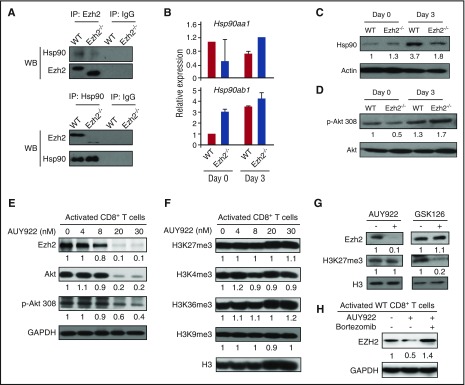
Figure 2.
Ezh2 and Hsp90 form a complex to stabilize Ezh2 protein in T cells. CD8+ T cells were isolated from B6 mice and stimulated with anti-CD3 antibody (3 μg/mL), anti-CD28 antibody (2 μg/mL), and recombinant murine interleukin-2 (5 ng/mL) for 3 days. (A) Cell lysates were prepared for coimmunoprecipitation (IP) assay using antibodies specific to Hsp90 and Ezh2. Western blots (WBs) show the levels of Ezh2 and Hsp90. (B) mRNA levels of Hsp90aa1 and Hsp90ab1 were detected in unstimulated (day 0) and activated (day 3) WT and Ezh2−/− T cells. (C-D) Protein levels of Hsp90, Akt, and phosphorylated Akt (p-Akt) in WT and Ezh2−/− T cells were determined by WB. (E-F) Activated T cells were treated with different doses of AUY922 for 16 hours. The expression levels of Ezh2, Akt, p-Akt, and histone methylation were examined. (G) Activated CD8+ T cells were treated with or without AUY922 (20 nM) or GSK126 (4 μM) for 48 hours. The levels of Ezh2 and H3K27me3 were determined. (H) Activated CD8+ T cells were treated with or without AUY922 (20 nM) for 48 hours. Bortezomib was added to the culture in the final 4 hours. The Ezh2 level was determined. Data are shown as mean ± standard deviation and representative of 2 to 4 independent experiments.