Figure 2.
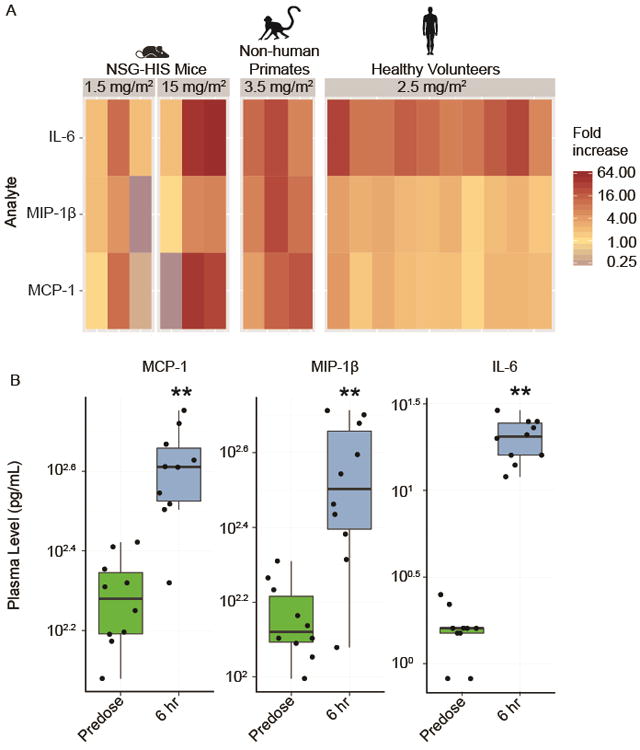
The NSG-HIS mouse model is suitable for the preclinical assessments of motolimod. (A) The in vivo plasma biomarker response to motolimod, administered at the respective doses shown, measured at 6 hours, is compared across NSG-HIS mice (n=3/group), non-human primates (n=3/group), and healthy human volunteers (n=10/group; 5 males and 5 females). Multiple cytokines and chemokines indicative of TLR8 stimulation and immune activation were evaluated using HumanMAP®. Representative analytes showing fold increase changes are shown. (B) In healthy volunteers (n=10), significant upregulation in several analytes is shown 6 hours after dosing with motolimod (p≤0.01, one-sided Wilcoxon signed-rank test) as denoted by asterisks (**).
