Figure 3.
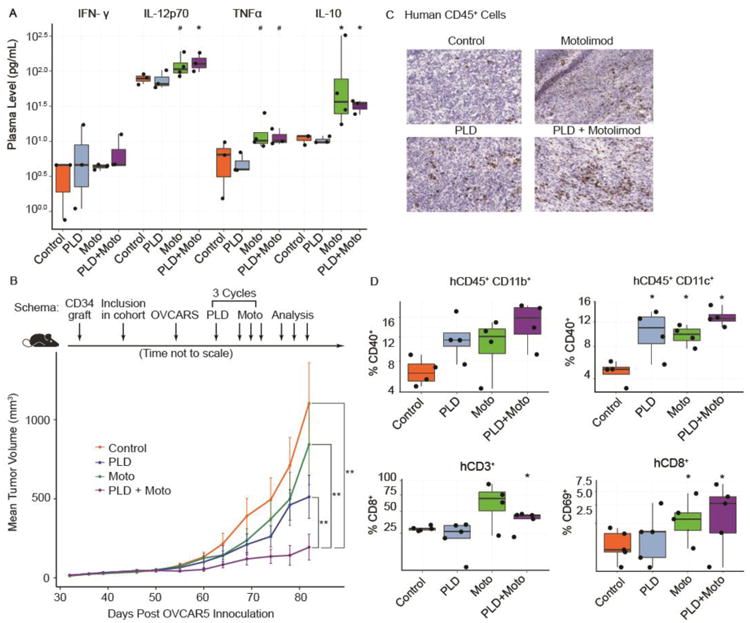
Preclinical studies of motolimod (Moto) plus pegylated liposomal doxorubicin (PLD) in NSG-HIS mice. (A) NSG-HIS mice were given either PLD alone (intraperitoneally, 50 mg/m2), motolimod alone (subcutaneously 2 days after PLD dosing, 1.5 mg/m2), the combination, or vehicle control. Plasma was collected 6 hours after dosing and levels of cytokines and chemokines were assessed using the HumanMAP® panel. Select biomarkers are shown. Symbols denote p-values compared to control (*=p≤0.05, #=p≤0.1; 1-sided Wilcoxon rank-sum test). (B) Tumor-bearing NSG-HIS mice inoculated with the human ovarian cancer cell line OVCAR5 were randomized to receive vehicle control, PLD, Moto, or the combination. Tumor growth was assessed at multiple time points post-inoculation. The combination of PLD + Moto showed highly significant improvement compared to control, PLD, and Moto. Two of the 10 mice in the PLD + Moto arm had no measurable tumors at the last 2 time points. Two asterisks (**) denote p≤0.01; 2-sided log-linear mixed-effect model. (C) IHC was used to assess the presence of human (h)CD45+ tumor infiltrating leukocytes. (D) The combination of PLD plus motolimod resulted in significant increases in hCD11b+ and hCD11c+ cells expressing hCD40 (top panel). The combination also yielded increases in the frequency of hCD8+ T cells expressing hCD69 (bottom panel). Treatment group(s) (n=5/group) with significant magnitude increase(s) with respect to the control (p≤0.05; 1-sided Wilcoxon rank-sum test) are identified with an asterisk (*).
