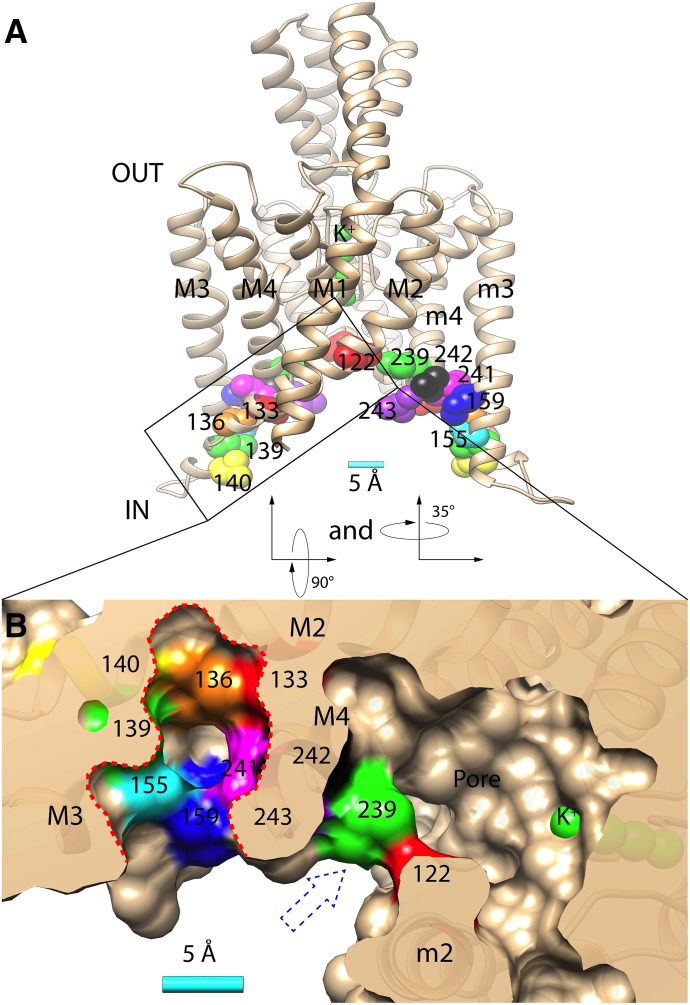
Fig. 1.
(A) Homology model of TASK-3 potassium channel (spanning residues Gln-4 to Val-243) with relevant residues indicated: Leu-122 (red), Met-133 (red), Val-136 (orange), Leu-139 (green), Leu-140 (yellow), Met-159 (blue), Leu-239 (green), Leu-241 (magenta), Leu-242 (black), Val-243 (purple), and four potassium ions in the selectivity filter (green). M1-4 and m2-4 indicate the transmembrane domains 1–4 and 2–4 in opposing TASK-3 subunits, respectively. (B) Bottom/cytoplasmic view achieved by rotating the structure in A first 90° then 35° along its x-axes and y-axes, respectively. The surface rendering is transected through the putative anesthetic binding pocket (perforated red line) and the pore fenestration (blue perforated arrow). A 5 Å (Å) scale bar is provided in A and B.