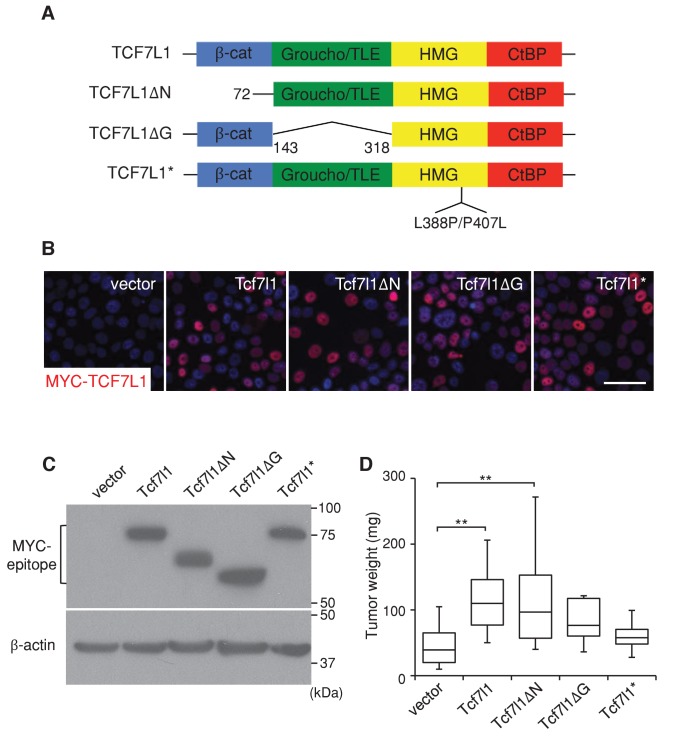
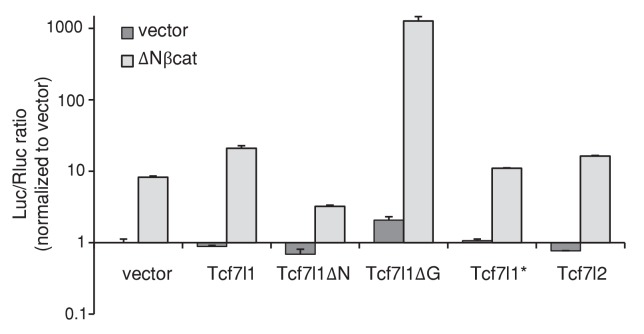
Figure 5. TCF7L1 promotes tumor growth independently of β-catenin binding.
The human skin SCC cell line SCC13 was transduced with pINDUCER21 lentiviral vectors expressing GFP alone or GFP with tet-inducible, myc-tagged murine Tcf7l1 or Tcf7l1 deletion mutants. After enrichment by FACS, the transduced cells were xenografted into flanks of immunodeficient mice, which were then put on a doxycycline-containing diet. (A) TCF7L1 and its mutant versions are schematized with amino acid deletion and point mutation annotated. (B) Immunofluorescence analysis of myc-TCF7L1 expression in cells overexpressing TCF7L1 or its mutants. Bar denotes 50 µm. (C) Western analysis of protein expression against myc epitope in transduced cells. (D) Graph quantifying the mass of tumors derived from SCC cells with vector or the overexpression of Tcf7l1 or its mutants. n = 12 (vector), n = 19 (Tcf7l1), n = 15 (Tcf7l1ΔN), n = 7 (Tcf7l1ΔG), n = 4 (Tcf7l1*). The grafted tumor mass data is presented as box and whisker plots where boxes span first and third quartiles, bars as the median values, and whiskers as minimum and maximum of all data **p<0.01 (One-way ANOVA with Dunnett’s post-hoc test).