Figure 5.
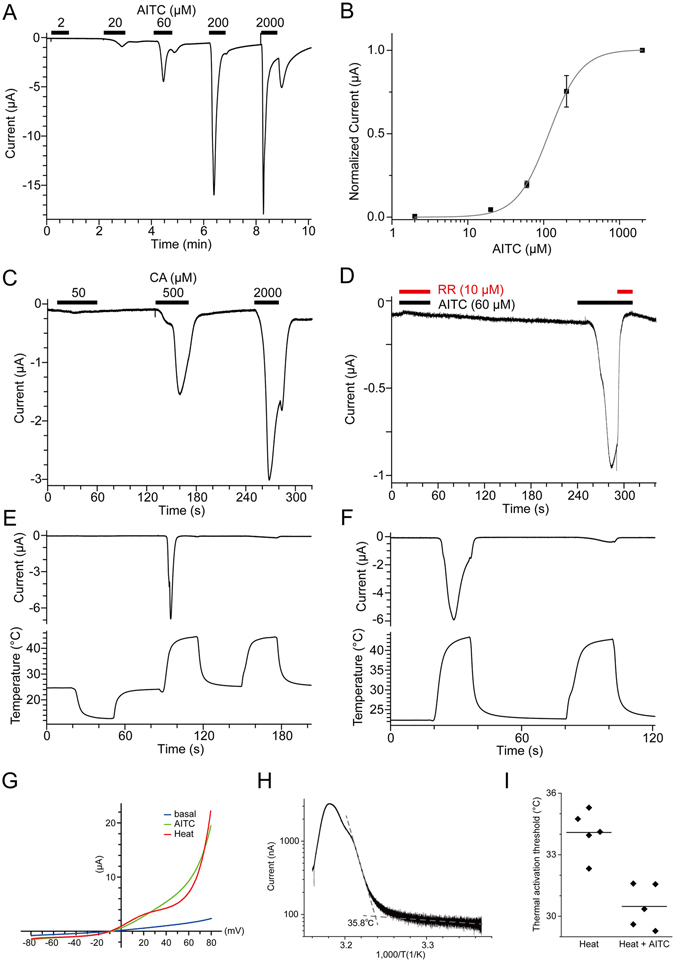
Activation of PpTRPA1 by heat and chemical stimulation. (A, B) A representative current trace against AITC stimulation in X. laevis oocytes expressing (A) PpTRPA1 and (B) its dose dependency (n = 7 or 9). (C) A representative current trace against cinnamaldehyde (CA) stimulation in X. laevis oocytes expressing PpTRPA1 (n = 3). (D) Inhibition of AITC-evoked currents by RR in X. laevis oocytes expressing PpTRPA1 (n = 4). Concomitant application of AITC and RR in the first stimulation completely suppressed the inward current, which was clearly observed in the second stimulation with AITC alone. (E, F) Representative current (upper) and temperature (lower) traces of thermal stimulation in PpTRPA1-expressing X. laevis oocytes (n = 4 and 11 for E and F, respectively). (G) Current-voltage relationships for heat and AITC (60 μM) stimulation in X. laevis oocytes expressing PpTRPA1. (H) An Arrhenius plot for the heat-evoked current in PpTRPA1-expressing X. laevis oocytes. The data obtained from the first heat stimulation, such as that shown in panel F, was used for analysis (n = 23). (I) Thermal activation thresholds for PpTRPA1 stimulated with or without AITC (10 μM). Thermal stimulation was applied in the presence or absence of AITC (10 μM) to X. laevis oocytes expressing PpTRPA1, and thermal activation thresholds of heat-evoked currents were compared using the same preparation (heat alone: 34.1 ± 0.5 °C, n = 5; heat with AITC; 30.5 ± 0.5 °C, n = 5; p < 0.01 with unpaired t-test). Each dot indicates the thermal activation threshold obtained from different oocytes. Average values are shown with bars.
