Figure 2.
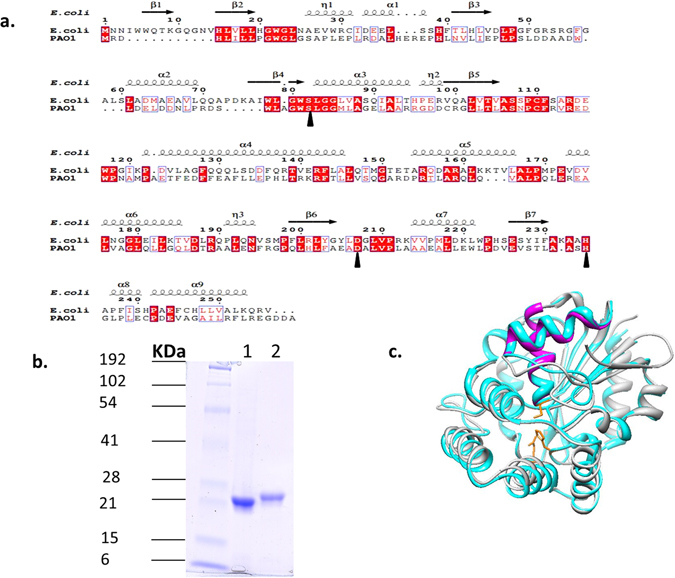
Sequence alignments, purification and modeling of BioH proteins. (A), sequence alignment of the BioH proteins of E. coli and P. aeruginosa PAO1. (a) Conserved residues are shown as white letters on a red background, and similar residues are shown as red letters in blue boxes. The E. coli BioH secondary structure (Protein Data Bank ID 1m33) is shown at the top of the panel. The catalytic triad residues are denoted by black arrow heads. (b) Purification of P. aeruginosa BioH (lane 1) and E. coli BioH (lane 2). The molecular masses of prestained broad-range protein standards (Bio-Rad) are indicated. The proteins were purified as described under Materials and Methods and analyzed by SDS-PAGE on a 15% polyacrylamide gel. (c) Structural model of P. aeruginosa BioH (cyan) obtained by threading on the structure of E. coli BioH (grey) using the SwissModel website and PDB 1m3317. Helices 2 and 3 of the lid domain of E. coli BioH are coloured magenta. The side chains of the catalytic triad residues are coloured yellow.
