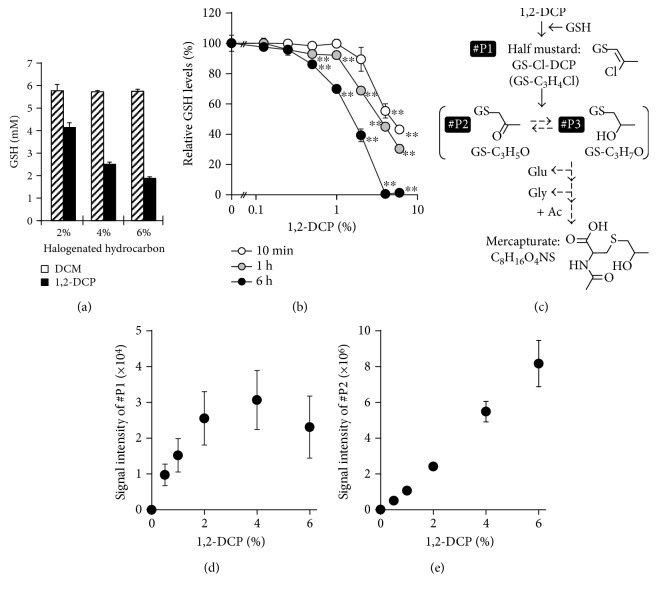
Figure 1.
Spontaneous reaction of 1,2-DCP with GSH under physiological pH conditions. (a) GSH levels in the reaction mixture containing halogenated hydrocarbons. Each in vitro mixture containing 6 mM GSH and DCM (shaded) or 1,2-DCP (black) at the indicated concentration (v/v) was incubated at 37 °C for 60 min, followed by determination of GSH concentration in the resulting solution. (b) Time- and dose-dependent decreases in GSH concentration in the presence of 1,2-DCP. The levels of remaining GSH under each experimental condition were normalized against the initial GSH concentration. Statistical analyses for significant differences were performed according to Bartlett's test, followed by Shirley-Williams' multiple-comparison test. ∗∗ P < 0.01 versus control in each group. (c) Putative metabolic pathway of 1,2-DCP and structures of relating metabolites in the presence of GSH. This metabolic scheme was modified from that of a previous report [12]. #P1 (GS-Cl-DCP) and #P2 represent the detected GSH-conjugated forms of 1,2-DCP, whereas #P3 was not detected in the present study. Dashed arrows mean that the processes were not observed in the present study. (d and e) Dose-dependent increases in GSH-conjugated forms of 1,2-DCP. Each mixture containing 6 mM GSH and 1,2-DCP at the indicated concentration (v/v) was incubated at 37 °C for 60 min, followed by examination of (d) GS-Cl-DCP (#P1) and (e) GS-DCP (#P2) levels in the resulting solution analyzed by LC-MS. Data were expressed as means ± SD, n = 4.