Fig. 1.
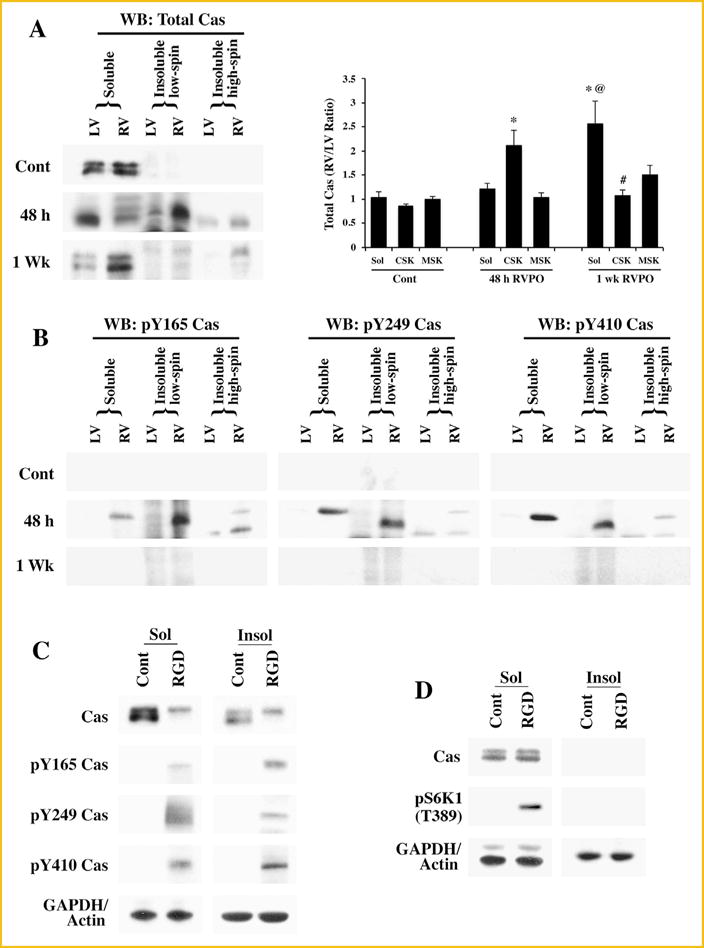
Subcellular localization and tyrosine phosphorylation of Cas: (A) Adult felines underwent pulmonary artery banding to produce RVPO and were sacrificed after the indicated time points. In addition to Sham operated animals that served as controls (Contl; LV or RV), the left ventricles (LV) in each experimental animal also served as internal normally loaded controls. The LV and RV samples from Sham control and PO samples for 48 h and 1 wk were processed to obtain Triton X-100 soluble (Sol) and insoluble (low spin and high spin) protein samples. Prior to the Western blot analyses on Cas, protein concentrations between LV and RV pairs for the soluble, insoluble low spin pellet (cytoskeleton) and the insoluble high spin pellet (membrane skeleton) were adjusted based on the GAPDH levels for the soluble samples and actin levels for the insoluble samples. The figure shows Western blot analyses for total Cas (left panel). For quantitation (right panel), Sham controls (n = 4), 48 h (n = 5) and 1 wk (n = 4) RVPO feline groups were scanned and RV to LV ratios for each fraction were determined and plotted. Values are the mean ± SEM. *P < 0.05 compared to control CSK; @P < 0.05 compared to Cont Sol or 48 h RVPO Sol; #P< 0.05 compared to 48 h CSK. (B) Triton X-100 soluble and insoluble fractions were used for Western blot with specific antibodies to detect tyrosine phosphorylated Cas at the Y165, Y249, and Y410 sites. Results were confirmed in at least two additional independent experiments (n = 3). (C) Adult cardiomyocytes were embedded within a 3D collagen matrix (3D) in the presence or absence of 9 mM RGD peptide for 1 h. After RGD treatment, cells were harvested for subcellular fractionation (Triton X-100 soluble and insoluble) and the protein samples were used for Western blot analyses of total and tyrosine phosphorylated Cas with specific antibodies as described in (A and B). Results were confirmed in two other independent experiments (n = 3). (D) Adult cardiomyocytes were cultured 2D on laminin-coated plates for 24 h and then stimulated with ± 9 mM RGD peptide for 1 h. Cells were harvested for subcellular fractionation (Triton X-100 soluble and insoluble) and the protein samples were used for Western blot analyses of total and tyrosine phosphorylated Cas with specific antibodies as described in (A and B). Results were confirmed in two other independent experiments (n = 3).
