Fig. 4.
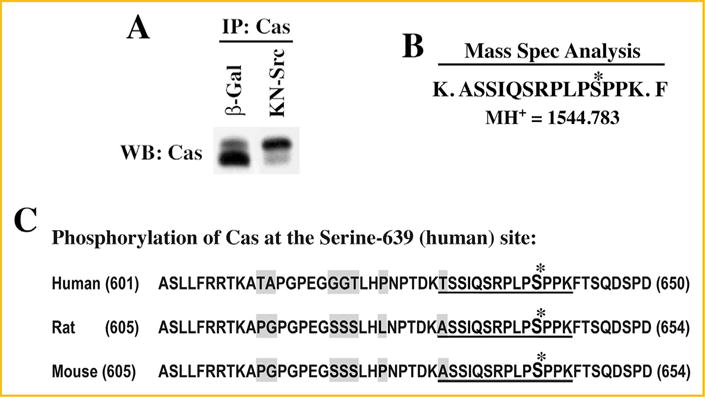
Mass spectrometry analysis of Cas phosphorylation: CHO cells were transfected with pcDNA6V5/His vector containing Cas cDNA (Rat) and selected for stably expressing cells with blasticidin resistance. (A) CHO cells stably overexpressing Cas were infected with β-gal and DN-Src adenoviruses for 36 h. Triton X-100 soluble samples were prepared and used for immunoprecipitation with anti-Cas antibody immobilized on agarose beads as detailed under Methods. A small part of the immunoprecipitated proteins were run on SDS–PAGE and used for Western blotting with anti-Cas antibody. (B) The remaining part of the immunoprecipitate run on SDS–PAGE was stained with Coomassie blue, and the region corresponding to the size (130 kDa) of Cas were cut, digested with trypsin and used for mass spectrometric analysis. A peptide with the molecular mass of 1,544.783 that corresponds to amino acids -ASSIQSRPLPSPPK- with phosphate group on the 4th serine residue was detected in KN-Src expressing cell extracts but not in β-gal control extracts. (C) Cas amino acid sequence flanking the phosphorylated serine residue is shown for human, rat, and mouse. The phosphorylated residue corresponds to serine-639 (human) and serine-643 (rat and mouse). The phosphorylated residue and the flanking amino acids identified in the mass spectrometry analysis are indicated with *mark and underline, respectively. The non-conserved residues among the species are highlighted in gray.
