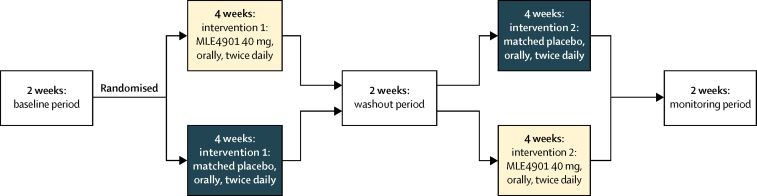
Figure 1.
Summary of protocol
Baseline period: participants underwent a 2 week period to gather baseline data on hot flush frequency, severity, bother, and interference (Hot Flash Related Daily Interference Scale). If the inclusion criteria regarding hot flush frequency and severity were met at the end of this period then they were assigned to the active phase of the study. Intervention 1 (double-blind): all participants randomly assigned to either 4 weeks of treatment with oral, twice daily 40 mg MLE4901 or exact-match placebo. Washout period: all participants underwent a 2 week washout period after intervention 1 (half-life of MLE4901 is 8·5 h). Intervention 2 (double-blind): all participants then switched to receive either 4 weeks of treatment with oral, twice daily exact-match placebo or oral, twice daily 40 mg MLE4901 depending on which intervention they received first. Monitoring period: a subsequent 2 week period to complete safety monitoring.