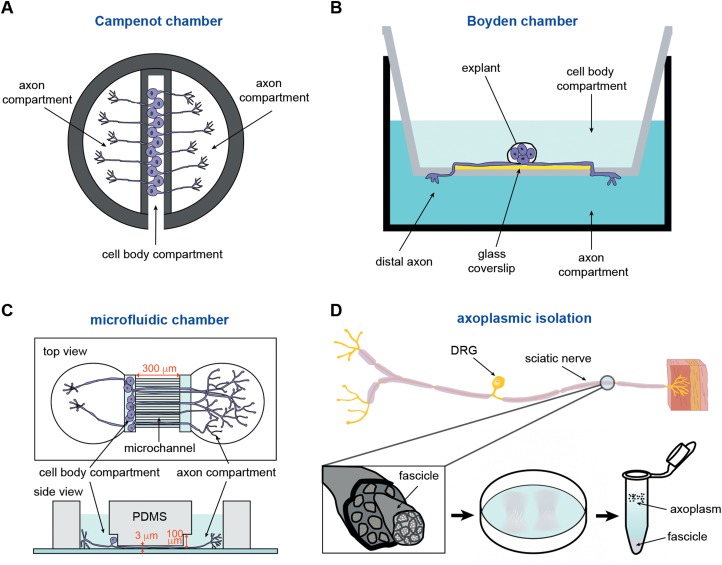
Figure 1.
Methods for isolating axonal miRNAs. (A) The Campenot chamber includes a scaffold made of Teflon, which is tightly adhered to a glass coverslip through silicone grease. The original three-chamber system consists of a central compartment and two side compartments. The dissociated neurons are plated in the central compartment. After several days in culture, only the long axons are able to pass through the silicone grease to both side compartments. (B) The Boyden chamber consists of a hollow plastic chamber sealed with a porous membrane containing pores of various sizes, allowing the motile cells to move to the other side. Explants or dissociated neurons are plated on a glass coverslip that is placed on the top of the microporous membrane. The growing axons cross through the membrane after several days. (C) The microfluidic chamber utilizes the replica-moulded transparent polydimethylsiloxane (PDMS) to establish a multi-compartment platform for cell culture. The chamber consists of separate compartments for the cell body and the axon, with microchannels (300 μm) linking the two compartments (top view). The cell body compartment is 100 μm high and is used for plating of neurons, whereas the microchannels are 3 μm in height and used for axon growth (side view). (D) The fascicles of axoplasm are mechanically separated from dissected sciatic nerve and incubated with a hypotonic solution (0.2× phosphate-buffered saline), either retaining intact axons or destroying Schwann cells. The ‘cloudy’ fascicles are incubated with the hypotonic solution for 2 h. After washing several times with the hypotonic solution, the axoplasm is eventually obtained in the supernatant of the solution (1× phosphate-buffered saline).