Fig. 1.
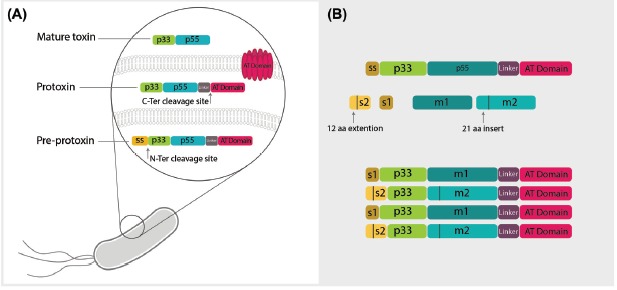
The molecular structure of vacuolating toxin of Helicobacter pylori. A) The vacuolating toxin of Helicobacter pylori is expressed as pre-protoxin inside bacteria containing p33 and p55 domains, a linker domain and autotransporter (AT) domain responsible for toxin secretion from bacteria. Maturation of the toxin occurs through two enzymatic cleavages. First, spontaneous N-ter cleavage site leads to breakage of signal sequence (ss), which is a determinant of directing of toxin to periplasmic space. Next, cleavage of the AT domain occurs simultaneously with the secretion of mature toxin to external environment of bacteria. The linker domain degrades by exposure to the environment and mature toxin is produced including two major regions known as p33 and p55. B) There are two diverse regions inside the mature toxin called s in p33 domain and m in p55 domain. The s region is located in the signal sequence of toxin in p33 with two types of s1 and s2 and an extra 21 amino acid extension sequence. The other divergent portion in p55 is also classified in two types, m1 and m2, with 21 amino acid insertion and shows different cell binding capacity to various receptors. Based on these two portions, VacA is classified into four groups of s1m1, s1m2, s2m2 and s2m1, all of which determine various pathogenicity based on their genotype.
