Fig. 2.
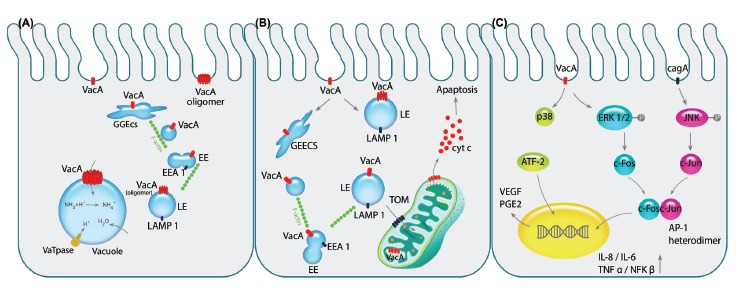
Pathogenicity of VacA toxin is exhibited on epithelial cells. A) After the pinocytosis of VacA into intracellular environment, it is directed towards early endosomal compartment GEEC, EE and LE respectively by the aid of F-actin filaments. Next, VacA is oligomerized on the LE membrane acting as the channel discharging chloride ions that results in LE swelling since water molecules are imported due to overpopulation of ammonium resulted from the change in chloride ions. Consequently, over-hydration results in vacuolation of the cell. B) Following aforementioned trafficking of the vacA to the endosomal compartments, its transportation to the mitochondria by N-ter signal sequence of the toxin can commence the programmed cell death (apoptosis). Importing of VacA through TOM complex and oligomerizing alters membrane potential of the mitochondria which recruit pro-apoptotic Bax and Bak complexes indirectly leading to release of cytochrome c. C) Binding of the VacA to cell surface receptor RPTPβ activates the MAPK signaling cascades independently or in cooperation with CagA. The activation of MAPK/p38 pathway induces stimulation of transcription factor ATF-2 that increases the expression of PGE2, known as the angiogenic factor and mitotic signal, via binding to COX-2 promoter. Additionally, VacA phosphorylates ERK1/2 that activates the downstream component c-fos, while CagA activates the other components of AP-1 heterodimer, c-jun, by phosphorylation of JNK. Interaction of c-fos and c-jun together would produce the AP-1 heterodimer which is counted as the transcription factor for several cytokines such as IL-1, IL-6 and TNFα leading to inflammation of the gastric mucosa.
