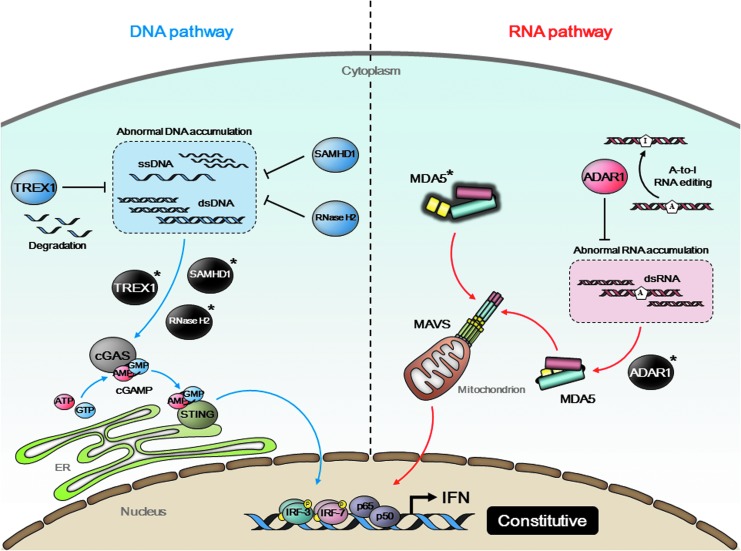
FIG. 2.
Type I interferonopathy caused by dysfunction of regulatory proteins. A cytoplasmic exonuclease TREX1 degrades extranuclear ss/dsDNA accumulated within intrinsic DNA metabolism. A dGTP-dependent triphosphohydrolase SAMHD1 and a heterotrimeric ribonuclease complex RNase H2 (RNase H2A, B, and C) also function as DNA metabolizing enzymes. Mutations in their respective genes (TREX1, SAMHD1, and RNASEH2A/B/C) abrogate their enzymatic activities and cause redundant accumulation of self-DNA in the cytoplasm. This abnormal DNA pool potentially stimulates cGAS-STING signaling axis (blue arrows). ADAR1, which is encoded by ADAR1, is an RNA-editing enzyme that binds to dsRNA and converts adenosine bases to inosines to prevent MDA5 from sensing endogenous dsRNA as nonself. Mutations in ADAR1 result in constant activation of MDA5 (red arrows). Gain-of-function mutation in IFIH1, which encodes MDA5, is also identified. All these mutations lead to constitutive activation of IFN and are strongly associated with Aicardi–Goutières syndrome (AGS), a genetic disorder referred to as type I interferonopathy.